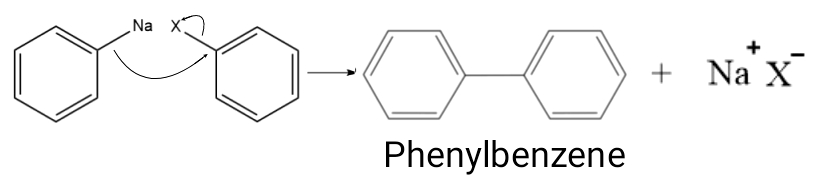
Fittig Reaction is a form of ‘Coupling Reaction’ in which two aryl(aromatic) groups combine in the presence of Sodium in dry ether or THF(Tetrahydrofuran) to form a biaryl species.
Example: Halobenzene reacts in the presence of sodium metal in dry ether to form biphenyl. Here, X = Cl, Br, I.
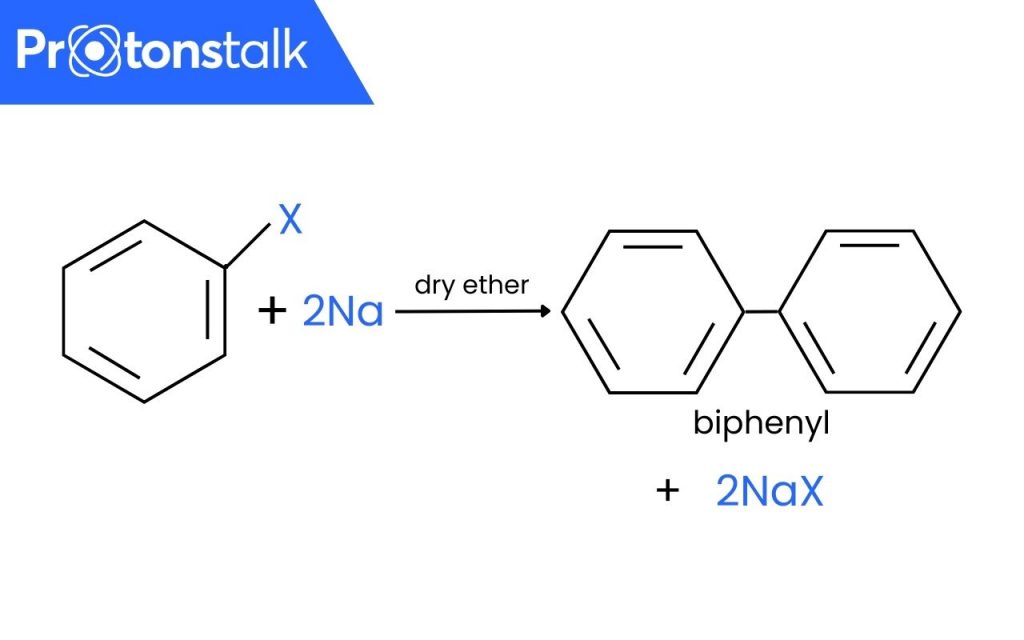
Although very similar but this reaction should not be confused with Wurtz-Fittig Reaction and Wurtz Reaction. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. Wurtz Reaction happens when two alkyl groups combine in the presence of Sodium metal in dry ether. While, in this reaction, two aryl groups combine with each other.
Related Topics:
Index
History

Wurtz Reaction was first reported by Charles Adolphe Wurtz in 1855.
Wilhelm Rudolph Fittig extended the work by Wurtz to include Aryl halides in the reaction. This gave rise to the Fittig Reaction and Wurtz-Fittig Reaction.
Fittig Reaction Mechanism
There are mainly two experimentally proven reaction pathways of the Fittig Reaction:
- Free Radical Mechanism
- The Organo-Alkali Mechanism
The Free Radical Mechanism
The free radical mechanism involves the formation of free phenyl radicals, which are highly reactive.
Step 1: Formation of Phenyl Free Radical
In the presence of Sodium in dry ether, homolysis of the C-X bond of the Aryl Halide happens. This leads to the formation of a phenyl-free radical and NaX salt.
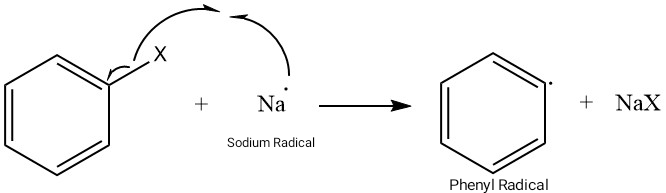
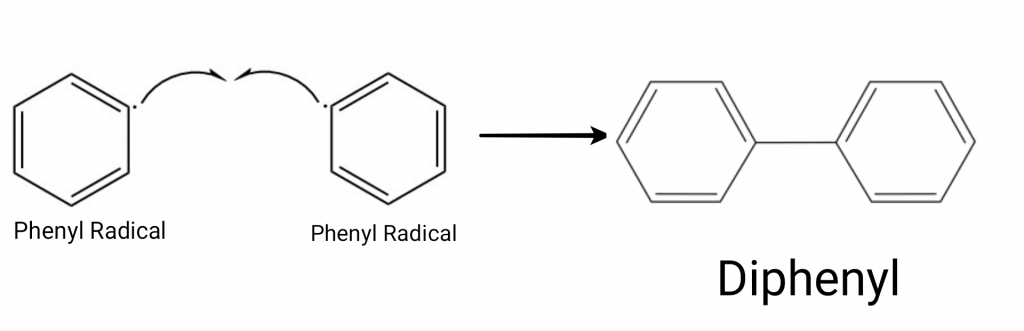
Step 2: Coupling of the Benzene Rings
The phenyl radicals formed in the previous step are highly reactive. Two phenyl radicals combine to form biphenyl(phenylbenzene or diphenyl).
The Organo-Alkali Mechanism
Another proven pathway to undergo this reaction is through the formation of an “Organo-Alkali” intermediate. Organic compounds form bonds with Alkali metals to form Organo-Alkali compounds.
Step 1: Formation of the Organo-Alkali Intermediate
Homolysis of the C-X bond in the Aryl Halide leads to the formation of phenyl radical, which combines with the sodium atom to form the Aryl-Sodium.
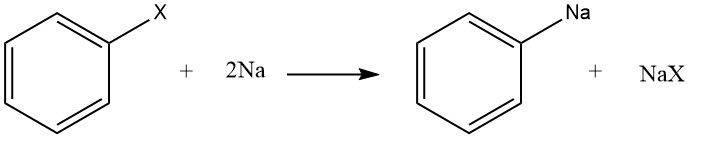
Step 2: Nucleophilic Attack of the Organo-Alkali to the Aryl Halide
Organo-Alkali is highly reactive in nature. The Aryl-Sodium here acts as a strong nucleophile and attacks the Aryl Halide present in the reaction mixture. This leads to the heterolysis of C-Na and C-X bonds. Na+ and X– combine to form a salt. Phenyl-benzene is formed as the product of this nucleophilic addition.
Applications of Fittig Reaction
This coupling reaction is not used in industry because of the reason that side-reactions like elimination and rearrangement are highly likely. Even then, this reaction is used in labs for the coupling of various aromatic rings in complex organic compounds.
Wurtz-Fittig reaction, a modification of this reaction, is used in labs to prepare Organo-Silicon compounds. It is also used for the Alkylation of Aryl Halides.
FAQs
This reaction is a form of ‘Coupling Reaction’ in which Aryl Halide reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce a bi-aryl compound and Sodium salt of the halide.
For example, Chlorobenzene reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce biphenyl and NaCl.
\(2C_6H_5Cl \ + \ 2Na \, \small{\text{(in dry ether)}} \rightarrow (C_6H_5)_2 \ + \ 2NaCl\)
Wurtz Reaction involves a reaction between Alkyl halides and Alkyl Halides.
Fittig Reaction involves a reaction between Aryl Halides and Aryl Halides.
Wurtz-Fittig Reaction involves a reaction between Aryl Halides and Alkyl Halides.
More Organic Reactions
