The Molisch test is a chemical analysis method utilized for identifying the presence of carbohydrates (monosaccharides, disaccharides, and polysaccharides) and glycoproteins in a given sample.
This test is useful in distinguishing between carbohydrates and proteins/amino acids.
The discovery of this Molisch’s test is attributed to Hans Molisch, a botanist from Czech-Austria who is its namesake.
Index
Explanation
The Molisch test is a highly sensitive colorimetric method used to detect the presence of carbohydrates, whether they are free or bound to proteins or lipids.
It involves using a solution of α-naphthol (C10H8OH) in 95% ethanol (C2H5OH), which is known as the Molisch reagent.
The formation of a purple or purplish-red ring at the site of contact between the H2SO4 and the analyte + Molisch’s reagent mixture indicates the presence of carbohydrates in the analyte.
So, the molisch’s test is also sometimes referred to as the purple ring test.

Note that if the concentrated acid is added too quickly, a black ring may form due to the heat generated from the reaction, which can carbonize/char the carbohydrates.
While all carbohydrates give a positive result with the Molisch test, exceptions include tetrose and triose.
Monosaccharides show a faster positive reaction compared to disaccharides and polysaccharides, which react slowly with the Molisch reagent.
Monosaccharides, also referred to as simple sugars, serve as the fundamental building blocks (monomers) of carbohydrates, representing the simplest form of sugar.
Disaccharides, also known as double sugars or biose, are formed when two monosaccharides are linked together by a glycosidic bond. Similar to monosaccharides, disaccharides are easily soluble in water. Sucrose, lactose, and maltose are three common types of disaccharides.
Polysaccharides, also known as poly-carbohydrates, are a type of carbohydrate commonly found in food. These complex molecules are made up of multiple monosaccharide units that are joined together by glycosidic bonds, resulting in long-chain polymers.
Overall, the Molisch test is a crucial tool for detecting the presence of carbohydrates in a given substance.
Principle of Molisch test
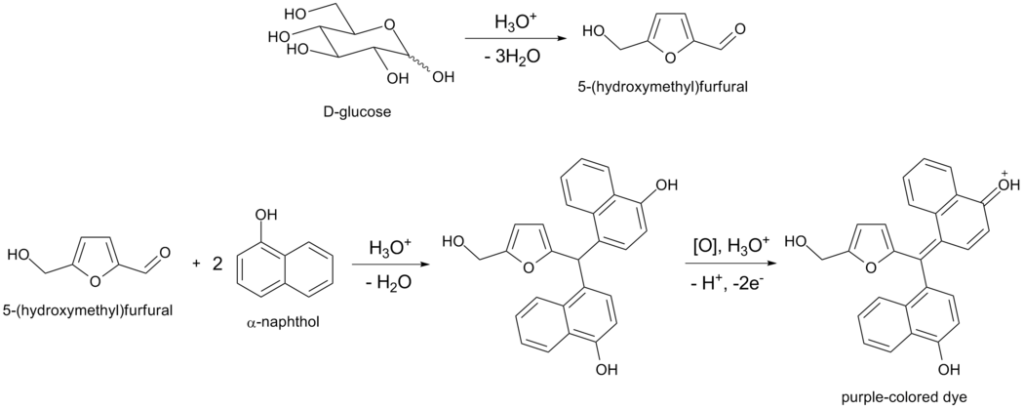
- The reaction involves concentrated acid catalyzing the dehydration of sugars to form furfural or hydroxymethylfurfural.
- One of these aldehydes reacts with two molecules of naphthol to form a purple or violet complex at the acid-test layer interface.
- If the carbohydrate is a polysaccharide or disaccharide, the acid hydrolyzes it into monosaccharides which further undergo dehydration to form furfural or its derivatives.
- Impurities in the reagent can lead to the formation of a green ring due to their interaction with α-naphthol (C10H8OH) and the acid (ethanol).
- A round ring may be observed if a concentrated sugar solution is used due to charring of the sugar by the acid (ethanol).
Requirements for Molisch Test
To perform the Molisch test, we will need the following materials:
- Test tubes and a test tube stand
- A pipette
- Distilled water
- Molisch reagent: A solution prepared by dissolving a solution of α-naphthol (C10H8OH) in 95% of ethanol (C2H5OH), which should always be freshly prepared.
- Concentrated sulphuric acid
- A test sample
Reaction of Molisch’s test
Here are the steps involved in the Molisch’s test reaction:
- Add concentrated sulfuric acid (H2SO4) to the analyte.
- The glycosidic linkage in sugar molecules, including disaccharides and polysaccharides, is hydrolyzed by H2SO4.
- Monosaccharides are formed as a result of the hydrolysis reaction.
- The hydroxyl (OH) groups in the sugar molecules are removed in the form of water due to the reaction with H2SO4.
- Furfural is produced from pentose sugar, and hydroxymethylfurfural is produced from hexose sugar (e.g., glucose).
- These reactive products react with α-naphthol.
- The reaction between the reactive products and α-naphthol (C10H8OH) results in the formation of a purple/violet colored complex.
Applications
- Molisch’s Test is utilized for identifying the presence of carbohydrates in different samples.
- One practical application of Molisch’s test is to determine whether a food product labeled as “sugar-free” actually contains carbohydrates or sugar.
This test can be used to quickly and easily confirm the presence or absence of such components in the product.
FAQ’S
It is a solution of α-naphthol (C10H8OH) in 95% ethanol (C2H5OH). It is used in a chemical test for the presence of carbohydrates.
Concentrated Sulfuric Acid (H2SO4) is used in the Molisch test.
The absence of five carbon atoms in triose and tetrose prevents the formation of furfural, leading to a negative result for the Molisch test reaction.
If the Molisch test result is negative, no purple-colored ring will form at the interface between the sulphuric acid and the test solution. Means the absence of a purple color indicates a negative result.
The Molisch test is utilized to differentiate carbohydrates from other biomolecules by detecting a by-product produced during various chemical reactions. It is a means to identify the presence of carbohydrates in a given sample.
Related Topics
