The Gattermann reaction is as an organic chemical reaction in which Aromatic compounds are formylated by a mixture of hydrogen cyanide and hydrochloric acid(HCL) in the presence of a Lewis acid catalyst such as Alcl3.
Formylation is a process where the formyl group(-CH=O) is attached to the compound.
It was named after a German chemist Ludwig Gattermann. It is also known as Gattermann formylation. It is a substitution reaction which is quite similar to the Friedel crafts reaction.
Index
Reaction
When Benzene or a derivative is treated with HCl and HCN in the presence of a Lewis acid catalyst (Alcl3), followed by hydrolysis gives benzaldehyde or substituted benzaldehyde.
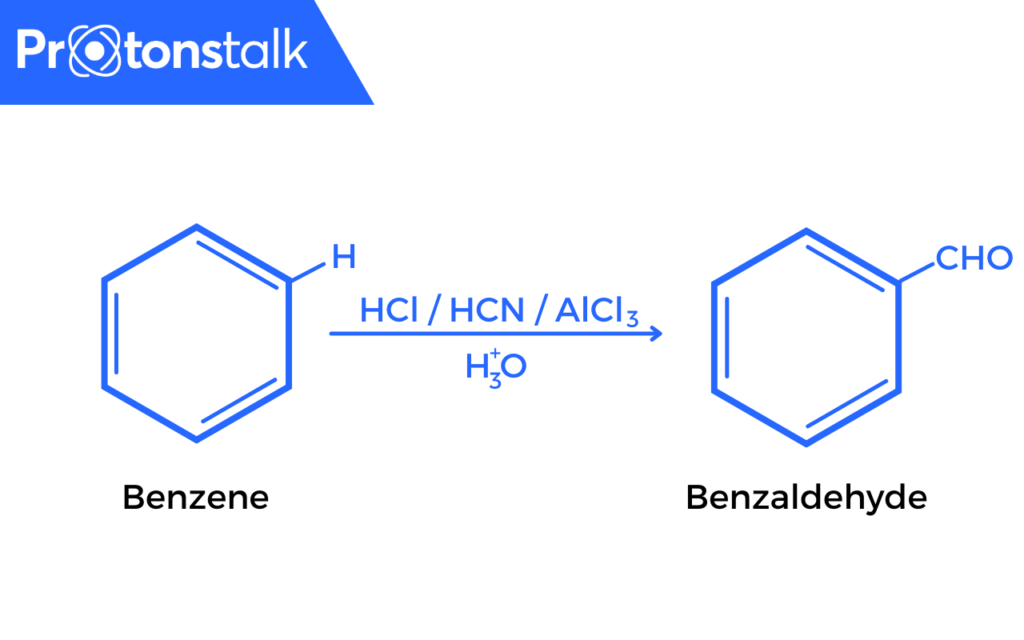
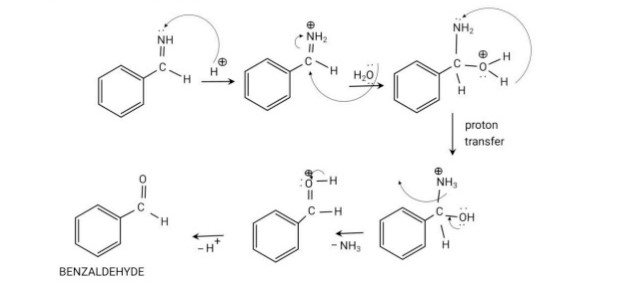
Gattermann Reaction Mechanism
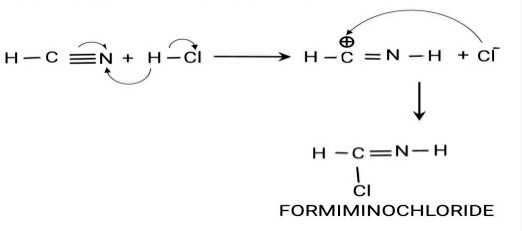
Step 1: Formation of Formimino Chloride: HCN Reacts with HCl to form formimino chloride.
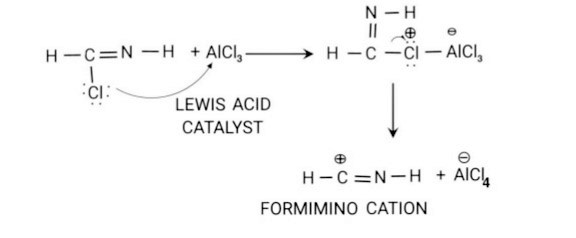
Step 2: Formation of Electrophile: Formimino chloride reacts with a lewis acid catalyst such as Alcl3 to form Formimino cation,
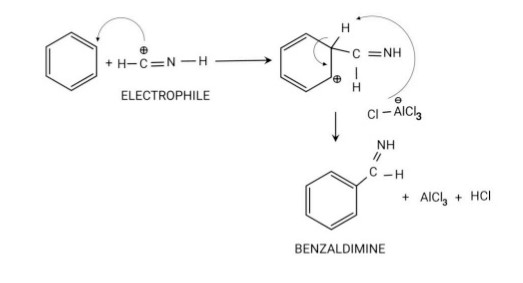
Step 3: Attack of Electrophile on Benzene Ring: The formimino Cation reacts with the benzene ring to form Benzaldimine.
Step 4: Hydrolysis of Benzaldimine: Hydrolysis of benzaldimine takes place which results in the formation of benzaldehyde.
Example
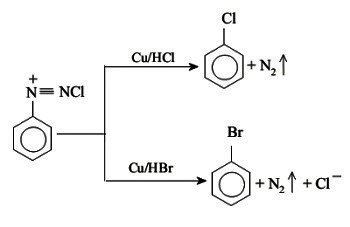
This reaction is used for obtaining chlorobenzene or bromobenzene from benzenediazonium chloride by treating it with Cu/HCl or Cu/HBr respectively.
Applications
- It is used in making aromatic halides such as Chlorobenzene and Bromobenzene.
- It is used in making aromatic aldehydes such as benzaldehyde.
- Products of this reaction are used in various fields such as pharmaceuticals, medicinal and Agriculture.
FAQs
Aluminium chloride(AlCl3).
Gattermann formylation and Gattermann salicylaldehyde synthesis.
Friedel craft’s reaction
More Organic Reactions
