Hund’s Rule or Hund’s Rule of Maximum Multiplicity is a rule in chemistry that deals with the pairing of electrons in energy orbitals.
We all know that Aufbau principle talks about how the lowest energy orbitals are filled first, and then move up to the higher energy orbitals. But, there is a problem with this rule. As we know, there are three p, five d and seven f orbitals in the respective subshells. This rule does not discuss the order of filling of these orbitals. This problem is resolved by Hund’s rule which was discovered in 1925, by Friedrich Hund.
Index
What is Hund‘s Rule?
The rule states that:
- Every orbital in a subshell is singly occupied before any orbital is doubly occupied.
- All electrons in singly occupied orbitals have the same spin.
Explaining Hund’s Rule
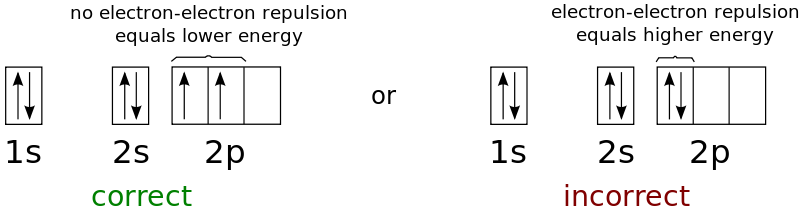
According to the first part of the rule, electrons always enter an empty orbital before they pair up. As electrons are negatively charged, they repel each other, thus they would want to be as far as possible at first.
According to the second part of the rule, unpaired electrons in the singly occupied orbitals have the same spin. Once the spin of the first electron is chosen, the spins of the remaining electrons in that sublevel depend upon the first electron’s spin.
In other words, the Hund’s rule of maximum multiplicity states that the greatest value of spin multiplicity has the lowest energy term. If two or more orbitals having the same energy levels are unoccupied then, electrons start occupying them individually before they are filled in pairs.
From Pauli’s exclusion principle, it can be realised that two electrons cannot share the same set of quantum numbers within the same system; there is room only for two electrons in each spatial orbital.
One of these electrons must have ms = ½ and the other, ms = -½. According to Hund’s Rule, the orbitals of the subshell are occupied singly with parallel-spin electrons, before double occupation occurs. Thus, the electrons present in singly occupied orbitals possess identical spin.
An Example
For example, a carbon atom has the electronic configuration 1s22s22p2. The same orbital is occupied by 2s electrons, whereas the different orbitals will be occupied by two 2p electrons.
FAQs
Hund’s rule of maximum multiplicity states that the greatest value of spin multiplicity has the lowest energy term. If two or more orbitals having the same energy levels are unoccupied then, electrons start occupying them individually before they are filled in pairs.
The Aufbau principle states that electrons fill lower energy orbitals, before filling higher energy orbitals.
Related Topics:
