Column Chromatography is a method in chemistry that is used to isolate a single chemical compound from a mixture. It is the ability to separate substances based on different adsorption of compounds to the adsorbent.
This method can be performed using gravity to move the solvent or using compressed gas to push the solvent through the column. The technique is applied on a small scale as well as a large scale.
Index
Column Chromatography Principle
The movement of individual components of the mixture is at different rates, when the mobile phase, along with the mixture that needs to be separated, is introduced from the top of the column.
Components having lower adsorption and affinity to the stationary phase travel faster when compared to the greater adsorption and affinity with the stationary phase.
The fast-moving components are removed first whereas the slower ones are eluted out last.
Adsorption of solute molecules occurs in a reversible manner. The rate of the movements of components is calculated as follows:
\(R_f = \frac{\text{Distance Travelled by Solute}}{\text{Distance Travelled by Solvent}}\)Where, \(R_f\) – Retardation Factor.
Phases in Column Chromatography
Now, let’s understand the different phases involved in column chromatography.
Mobile Phase: Made up of solvents. Has the following functions:
- Acts as a solvent – sample mixture introduced in column.
- Acts as a developing agent – helps in separation of components, to form bands.
- Acts as an eluting agent.
- Examples are ethanol, acetone, water, etc.
Stationary Phase: A solid material with good adsorption property. It should meet the following conditions:
- Shape: Uniform
- Size: 60 – 200μ
- High mechanical stability, and chemically inert.
- Should be colorless, inexpensive, and readily available.
- Should allow free flow of the mobile phase.
- Should be suitable for the separation of mixtures.
Column Chromatography Procedure
- A solvent is used to wet the stationary phase as the upper level of the mobile phase and the stationary phase should match. The compound mixture that is to be separated is added from the top of the column without disturbing the top level.
- The solvent mixture is added slowly by touching the sides of the glass column, without disturbing the stationary phase. The solvent is added throughout the experiment.
- The tap is turned on, thus initiating the adsorption process on the surface of silica. The compounds start to move in the mixture. This movement is based on the polarity of the molecules in the sample, non-polar components move at a greater speed compared to the polar ones.
- The lower polar compounds that arrive first at the end of the column are collected in a clean test tube.
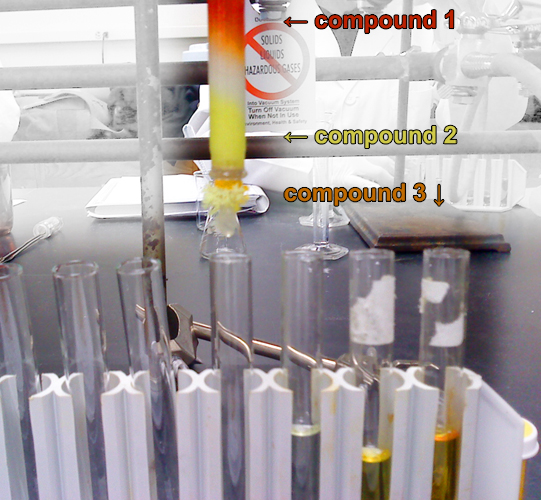
Applications
- One application of column chromatography is to isolate active ingredients.
- It is also helpful in separating compound mixtures.
- It is also used to determine drug estimation from drug formulations.
- This method is also used to remove impurities and also used to isolate metabolites from biological fluids.
FAQs
Adsorption is the principle behind column chromatography, in which a mixture of components dissolved in the mobile phase is introduced into the column and the components move depending on their relative affinities.
Silica gel and alumina are two examples of stationary phases and organic solvents are examples of mobile phases.
– One application of this method is to isolate active ingredients.
– It is also helpful in separating compound mixtures.
– It is also used to determine drug estimation from drug formulations.
– Column chromatography is also used to remove impurities and also used to isolate metabolites from biological fluids.
Related Topic: Difference Between Mixture and Compound
