Swarts Reaction is an organic reaction in which alkyl chlorides/alkyl bromides are usually converted to alkyl fluorides. This process was described by Frederick Jean Edmond Swarts in 1892.
This reaction is done by heating the alkyl chloride/alkyl bromide in the presence of the fluoride of heavy metals such as AgF, Hg2F2, CoF2 or SbF3.
The swarts reaction is also known as swarts fluorination.
Given below is an example :
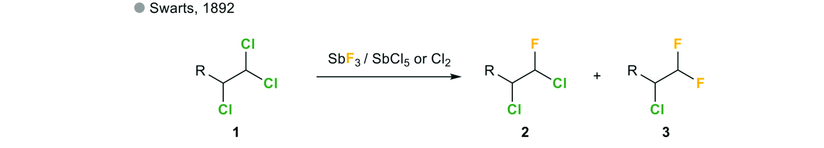
This reaction is usually used to replace chlorines by fluorines using antimony trifluoride (SbF3) in the presence of Sb salts in which antimony displays an oxidation state of +5 (SbCl5)
The mixture of antimony trifluoride(SbF3) and chlorine(Cl2) is known as Swarts reagent.
Index
Mechanism
The mechanism of Swarts Reaction is pretty simple and easy. (It basically follows SN2 reaction mechanism). The metal-fluorine bond is broken and a new bond is formed between carbon and fluorine. The displaced chlorine/bromine atoms now bond with the metal.
Applications
- This reaction is used to form alkyl fluorides.
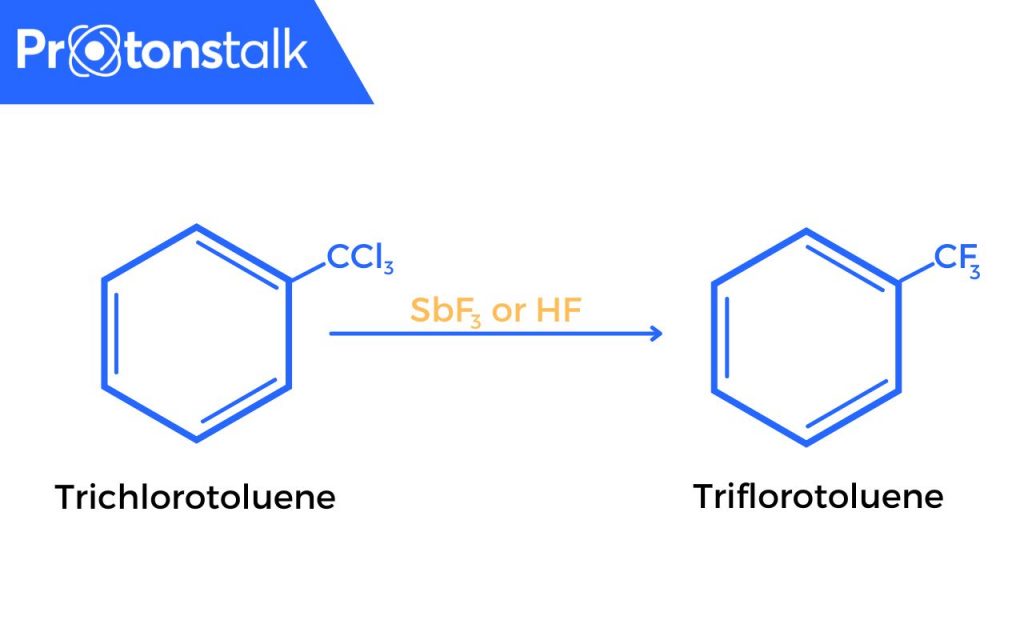
- A variant of this reaction is used to produce Freons. Freons are fluorinated aliphatic organic compounds. In this variant, fluorination is done using anhydrous hydrogen fluoride (HF) in the presence of Sb salts in which Sb displays oxidation states of + 3 and + 5
Finkelstein Reaction Vs Swarts Reaction
These two reactions are halogen exchange reactions related to alkyl halides. Swarts reaction converts alkyl chlorides/alkyl bromides to alkyl fluorides. Finkelstein reaction is classically used to convert alkyl chlorides/alkyl bromides to alkyl iodides.
FAQs
The mixture of antimony trifluoride(SbF3) and chlorine(Cl2) is known as Swarts reagent.
The reaction is an SN2 reaction in which one halogen atom is replaced by another halogen atom.
More Organic Reaction

Pingback: SN2 Reaction - Mechanism, Characteristics, Factors | ProtonsTalk