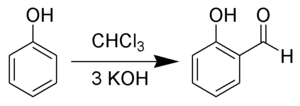
The Reimer Tiemann Reaction is an organic chemical reaction which is used for the ortho-formylation of the phenols(C6H5OH). It is basically substitution reaction and is named after Karl Reimer and Ferdinand Tiemann.
Ortho-formylation is a chemical reaction in which one hydrogen atom from the ortho position of the phenol, is replaced by a formyl group.
The conversion of phenol to salicylaldehyde is one of the common examples of Reimer Tiemann reaction.
Index
Ideal Reaction Conditions
- As hydroxides are generally not readily soluble in chloroform, the reaction is generally carried out in a biphasic solvent system. A biphasic mixture is a combination of two immiscible phases which usually consists of an organic solvent and an aqueous phase.
- The two reagents are therefore separated and must be brought together for the reaction to take place. These reagents are brought together by rapid mixing, phase transfer catalysts or the use of an emulsifying agent (the use of 1,4-Dioxane as a solvent is an example).
- The reaction will be effective when other hydroxy-aromatic compounds such as the naphthols are used.
- Heat is necessary to initiate the process of the reaction.
- Once the reaction is begun, it tends to be highly exothermic. As a result, the reaction is prone to thermal runaways (the exothermic reaction goes out of control).
Mechanism of Reimer Tiemann Reaction
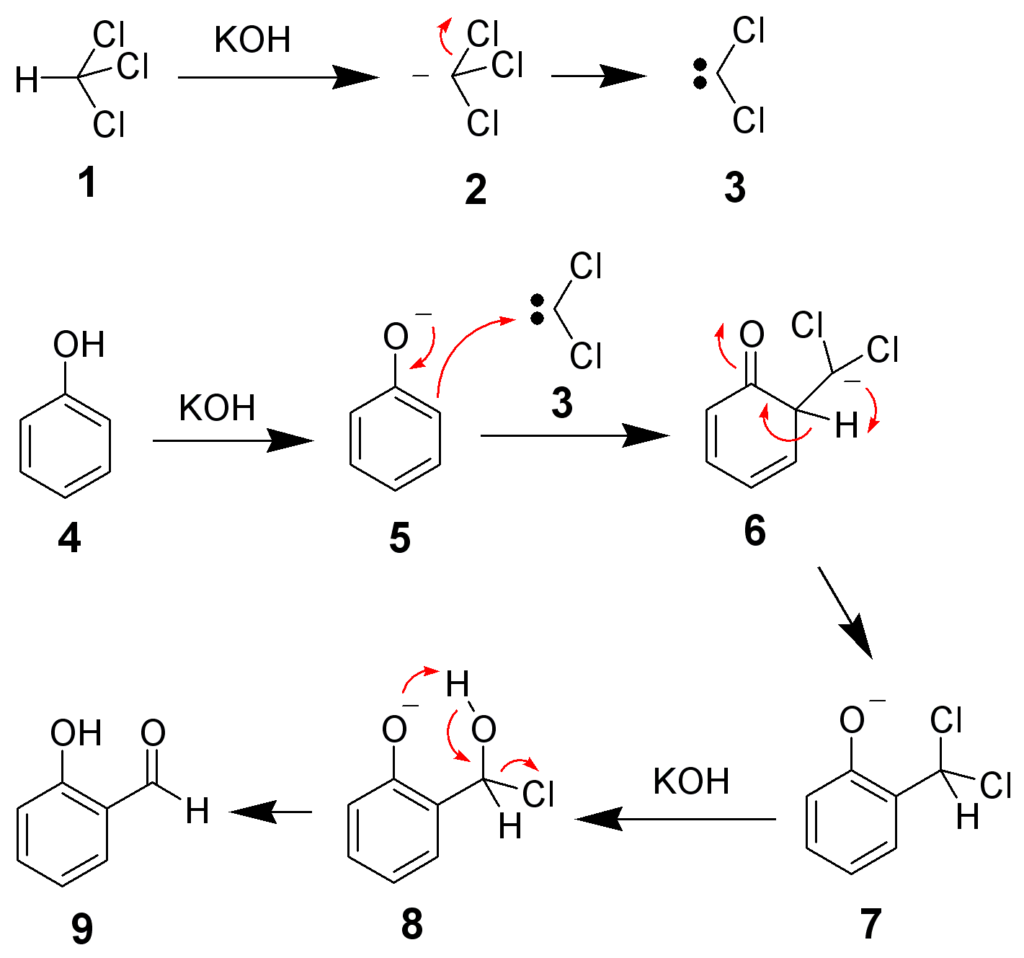
Firstly, the deprotonation of the chloroform takes place by a strongly basic hydroxide solution. In this case, Deprotonation is the removal of the hydrogen atom. Due to the removal of the hydrogen atom, we obtain the chloroform carbanion.
The chloroform carbanion will rapidly undergo alpha elimination to give dichlorocarbene(CCL2) which is the main reactive species of the reaction.
The phenol reactant is deprotonated by the aqueous hydroxide which leads to a negatively charged phenoxide.
The negative charge of the phenoxide is delocalised into the aromatic ring, making it more nucleophilic.
This leads to a nucleophilic attack on the dichlorocarbene and hence forming an intermediate dichloromethyl substituted phenol.
The obtained intermediate is subjected to basic hydrolysis to finally achieve the ortho-hydroxybenzaldehyde.
In the above diagram we should note that,
- Due to its 2 electron-withdrawing chlorine groups, carbene (3) is highly electron-deficient and is attracted to the electron-rich phenoxide (5). This interaction favours selective ortho-formylation.
- The Reaction involves Dichloro-carbene(3) as an electrophile.
Applications of Reimer Tiemann Reaction
- This reaction can be slightly altered to yield phenolic acids such as salicylic acid, by substituting the chloroform with carbon tetrachloride.
- It is mainly used for the ortho-formylation of the phenols.
- The direct formylation of the aromatic compounds can be done by various methods, but the safest and the easiest way is through this reaction, as it is the only method that does not require acidic or anhydrous conditions.
FAQs
Ortho position will be more electron-rich as compared to the para position due to the inductive effect, and the incoming electrophile (carbene) will attack the ortho carbon. Hence, ortho isomer is the major product.
Yes, Reimer Tiemann reaction is an electrophilic substitution reaction. The dichloro-carbene acts as an electrophile.
More Organic Reactions

I like the valuable information you supply for your articles.
I will bookmark your weblog and take
a look at once more here frequently.
I am reasonably sure I
will learn plenty of new stuff right right here!
Best of luck for the next!
Glad that you found it valuable!!