Wurtz Reaction is an organic coupling reaction in organic and organo-metallic chemistry which is named after Charles Adolphe Wurtz .
This reaction is also known as Wurtz coupling. It is a method used to synthesize higher alkanes by a reaction between alkyl halides and metallic sodium in the presence of dry ether.
Apart from sodium, metals like silver, indium, activated copper, zinc, and iron can also be used in this reaction in order to obtain alkanes.
This reaction occurs through a free radical mechanism that makes possible side reactions producing alkene as products.
Index
Wurtz Reaction Mechanism
The general form of reaction is as follows:
2 R–X + 2 Na → R–R + 2 Na+X−
From the above equation we observe that the 2 ‘R’ groups are joined resulting in a long alkane.
This reaction is a nucleophilic substitution reaction. The reaction consists of a halogen metal exchange involving radical Species R• .
The mechanism of this reaction is as follow:
Step 1. One electron from the metal is transferred to the halogen to produce a metal halide and an alkyl radical.
R–X + M → R• + M+X−
Step 2. The alkyl radical then accepts an electron from another metal atom to form an alkyl anion. This intermediate has been isolated in several cases.
R• + M → R−M+
Step 3. The nucleophilic carbon of the alkyl anion then displaces the halide in an SN2 reaction, forming a new covalent bond with carbon which was bonded with the halogen.
R–M+ + R–X → R–R + M+X–
As discussed earlier, the free radical mechanism of this reaction involves the possibility of an alkene being a side product.
The side reaction is as follows :
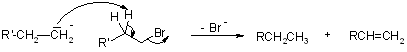
Limitations of Wurtz Reaction
- The reaction has relatively low yields due to the formation of multiple products.
- The Wurtz coupling method generally fails when tertiary alkyl halides are used.
- Methane can not be obtained by this method as the products of a coupling reaction should have at least 2 carbon atoms.
- Only symmetric alkanes can be synthesized via this method.
- If two dissimilar alkyl halides are taken as reactants, then the product is a mixture of alkanes that is often difficult to separate by fractional distillation.
Related
1. Wurtz Fittig Reaction
2. Fittig Reaction
Uses of Wurtz Reaction
- This reaction is used in closing small, especially 3 membered rings.
- Preparation of Bicyclobutane using the Wurtz coupling. The reaction is as follows:
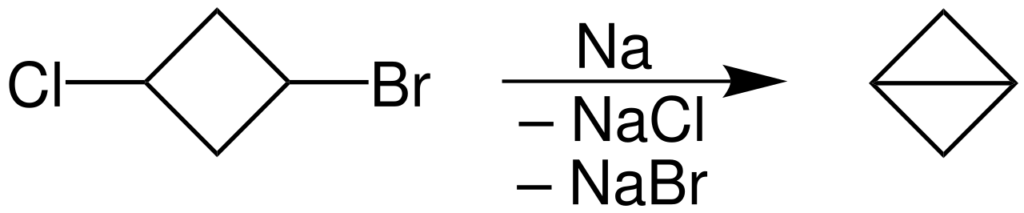
- This reaction gives alkenes as a product when the reactant is vicinal dihalide , and gives alkynes as a product if the reactant is Geminal dihalide.
Some examples of this reaction are given below,
CH3Br + 2Na + BrCH3 → C2H6 + 2NaBr
CH3CH2Br + 2Na + BrCH2CH3 → CH3CH2CH2CH3 + 2 NaBr
FAQs
Methane, as it contains only one carbon atom. The products of Wurtz reaction have a minimum of 2 carbon atoms.
The main disadvantage of “Wurtz reaction” is, it is not an ideal method for preparing alkanes because it undergoes various side reactions which results in less yield of the desired product.
The reaction condition requires an aprotic solvent as the medium of the reaction. Dry ether is a very good non-polar, aprotic solvent for this purpose. Hence it is used in Wurtz reaction.
More Organic Reactions
