The Haloform Reaction is an organic chemical reaction where a haloform (CHX3, where X is a halogen) is produced by the exhaustive halogenation of a methyl ketone (RCOCH3, where R can be either a hydrogen atom, an alkyl or an aryl group), in the presence of a base.
An example of the this reaction is given below:
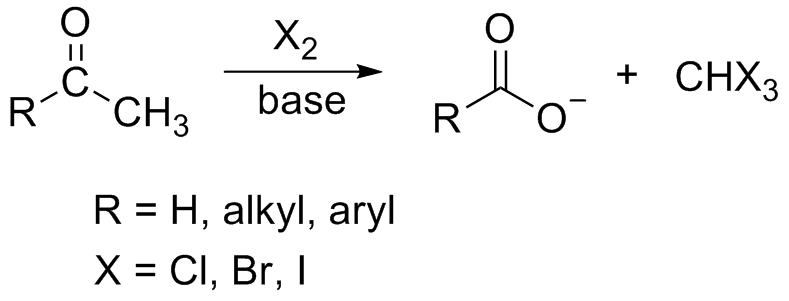
This reaction is a substitution reaction. This reaction is one of the oldest organic reactions and has a long history. Though it was independently discovered by various scientists, the reaction was rediscovered by Adolf Lieben in 1870. Thus, the iodoform test is also called the Lieben haloform reaction.
A review of the the reaction with a history section was published in 1934.
Index
Haloform Reaction Mechanism
In the first step, the halogen dis-proportionates in the presence of hydroxide to give the halide and hypohalite.
Br2 + 2OH─ → Br─+ BrO─ + H2O
We can classify the mechanism into 3 steps:
Step 1
The base (hydroxide ion) takes out the alpha hydrogen-producing an enolate. Then, the reaction between the enolate and the halogen occurs. Leading to the formation of the halogenated ketone along with the halogens corresponding anion. This step is repeated twice to yield a tri-halogenated ketone.
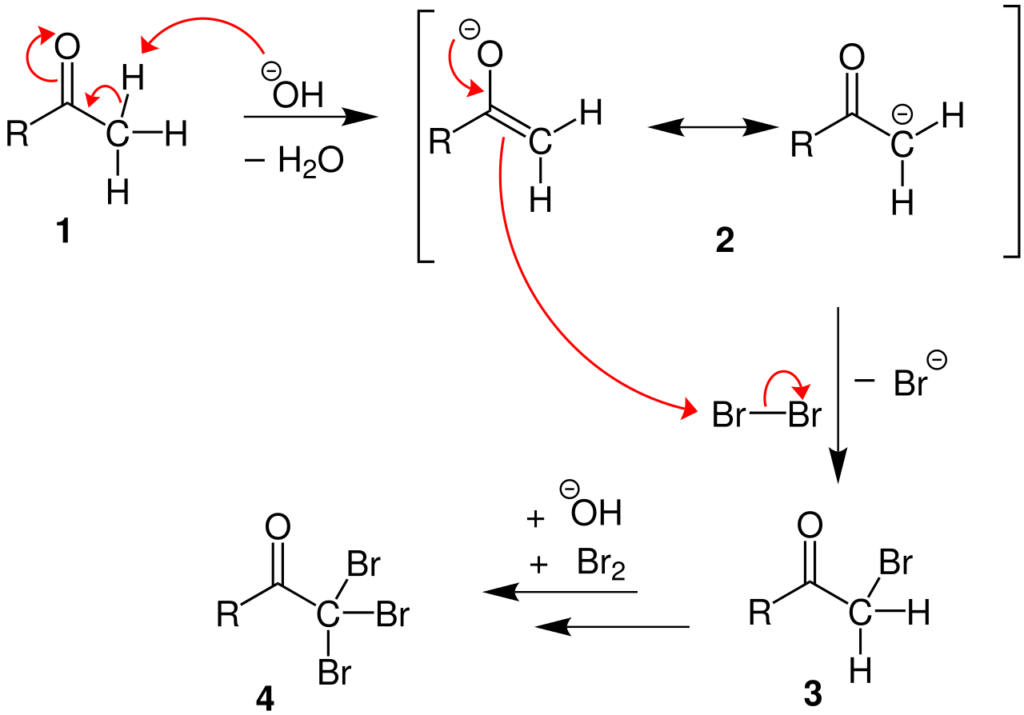
Step 2
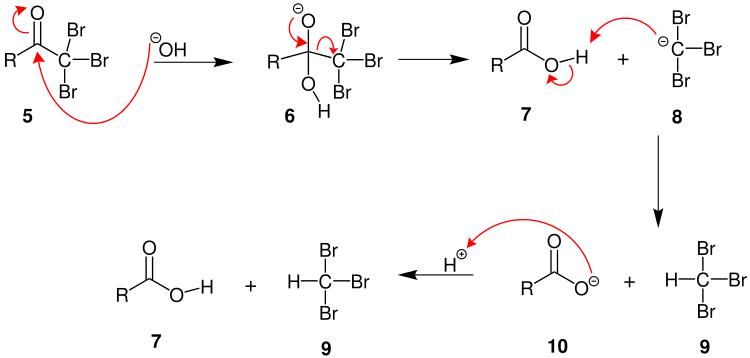
When the α(alpha) position has been exhaustively halogenated, the molecule undergoes a nucleophilic acyl substitution by hydroxide, with −CX3 being the leaving group stabilized by three electron-withdrawing groups.
Step 3
The −CX3 anion abstracts a proton from either the solvent or the carboxylic acid formed in the previous step, and forms the haloform. In some cases the reaction may stop and the intermediate product is isolated. If the conditions are acidic and a hypohalite is used then a chloral hydrate (-C2H3Cl3O2) is formed as an intermediate.
Applications of Haloform Reaction
- The reaction can be used to transform acetyl groups into carboxyl groups or to produce chloroform(CHCl3), bromoform(CHBr3), or iodoform(CHI3).
- It was formerly used to produce iodoform, bromoform, and even chloroform on an industrial scale.
- This reaction is used as a chemical test to determine the presence of a methyl ketone, or secondary alcohol oxidizable to a methyl ketone.
FAQs
Ethanol
A chemical reaction in which a methyl ketone is oxidized to a carboxylate by reaction with aqueous HO– and I2. The reaction also produces iodoform (CHI3), a yellow solid which may precipitate from the reaction mixture.
The reaction of a methyl ketone with chlorine, bromine, or iodine in the presence of hydroxide ions to give a carboxylate ion and a haloform is the haloform reaction.
A hypohalite is an oxyanion(XOー: where X is the halogen) containing a halogen in oxidation state +1. This includes hypoiodite, hypobromite and hypochlorite.
Related Articles:
