The Finkelstein Reaction is an organic SN2 Reaction(substitution nucleophilic bimolecular reaction) which is named after the German chemist Hans Finkelstein. This reaction involves the exchange of one halogen atom for another. Usually, it is used to convert alkyl chlorides/alkyl bromides to alkyl iodides.
The basic example of the reaction is given below:
R–X + X′− ⇌ R–X′ + X−
If this reaction is carried out without a solvent, the reaction is an equilibrium reaction.
Index
Mechanism
As mentioned the reaction follows SN2 reaction mechanism. The Finkelstein reaction classically involves the conversion of Alkyl bromides or Alkyl chlorides into Alkyl iodides by the treatment with a solution of Sodium iodide(NaI) in Acetone(C3H6O).
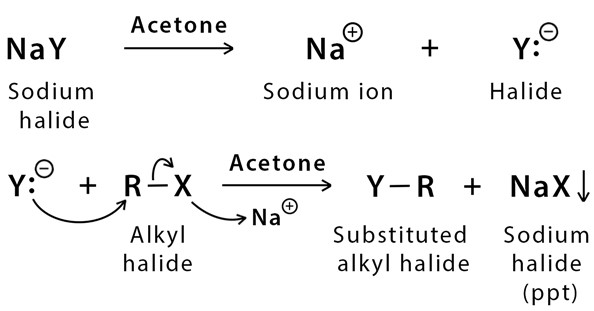
The mechanism of this reaction is easy and simple. It is a single step SN2 reaction. So, its mechanism is the same as the SN2 mechanism which occurs with the inversion of stereochemistry.
It is an equilibrium process and the forward reaction is supported by taking advantage of the poor solubility of the newly formed metal halide (sodium chloride or sodium bromide) salt in Acetone.
The sodium chloride and sodium bromide are not soluble in acetone but sodium iodide is soluble in acetone.
The reaction works good with the primary halides but better with \(\alpha\)-carbonyl halides and allyl benzyl. Secondary halides are far less reactive.
Applications
- It is used in the analysis of alkyl halides, as alkyl halides greatly differ in the ease with which they undergo the reaction.
- Used in easy production of alkyl iodides.
- Used in the synthesis of Chrysochlamic Acid.
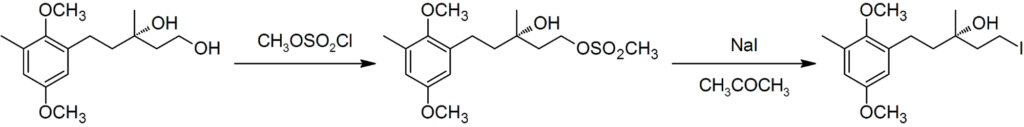
- The reaction of NaI in acetone can be used as a qualitative test to determine the class of an unknown halide .
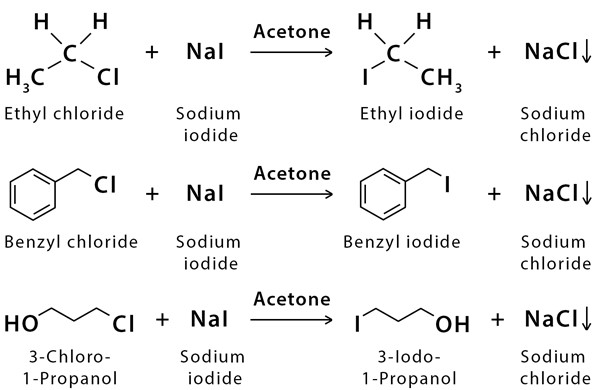
Examples
Given below are some examples of this reaction.
FAQs
Acetone is an organic solvent which is widely used in organic reaction. It is used in this reaction to facilitate the reaction in forward direction. According to the Le-chatelier’s principle, the reaction will move in that direction where the one of the reagents goes outside the solution.
Finkelstein reaction is SN2 reaction(substitution nucleophilic bimolecular reaction).
It is an SN2 reaction in which one halogen atom (the leaving group) is replaced by another halogen atom (the nucleophile).
Example:
Conversion of the ethyl ester of 5-bromovaleric acid to the iodide.
EtO2C(CH2)4Br + NaI → EtO2C(CH2)4I + NaBr
[Et =ethyl group]
More Organic Reactions

Pingback: Swarts Reaction - Mechanism, Application & Example | ProtonsTalk