Sandmeyer Reaction is basically a substitution reaction, which is widely used to synthesize aryl halides from aryl diazonium salts.
To be specific, this reaction is a type of Radical-nucleophilic aromatic substitution reaction. The catalysts used in this reaction are copper salts like bromide, chloride or Iodide ions.
Index
History
The reaction was first discovered in 1884 by a Swiss chemist Traugott Sandmeyer while he was conducting an experiment to synthesize phenylacetylene(C8H6) from benzene diazonium chloride(C6H5CLN2) and Cuprous acetylide(C2CU2). But instead, he obtained phenyl chloride as the main product.
Mechanism Of Sandmeyer Reaction
The Sandmeyer Reaction is a very important transformation in aromatic chemistry, because it can result in some substitution patterns that are not achievable by direct substitution.
This reaction on an overall has two different steps
- Formation of diazonium salts from the amine group attached to an aromatic ring. (Diazotisation)
- Transformation of diazo intermediates into aryl halides. This takes place in the presence of a nucleophile which can be a halide anion, cyanide, water etc.
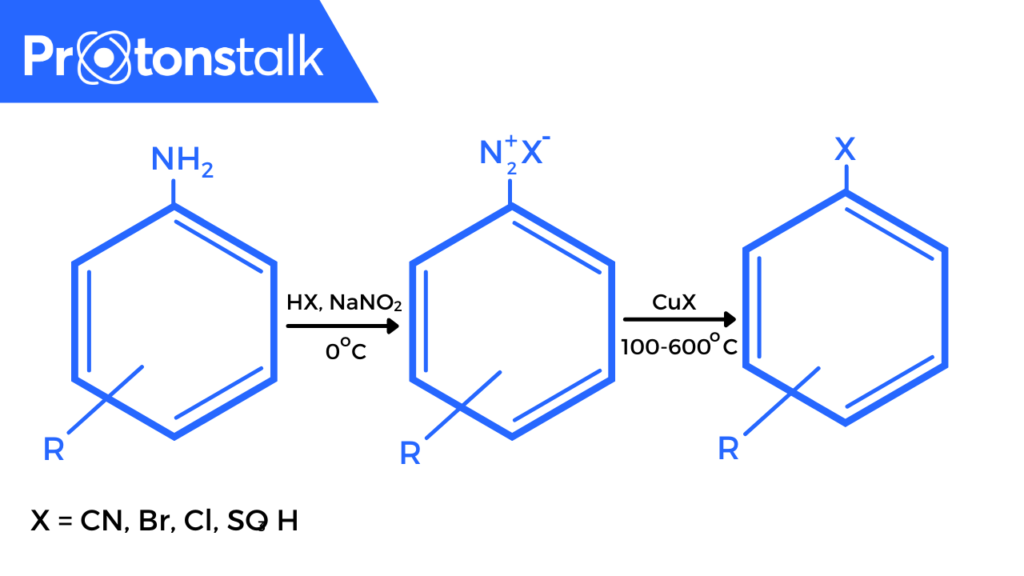
Figure 1: Sandmeyer Reaction
Sandmeyer reaction actually begins with a transfer of a single electron from copper (catalyst) to the diazonium. This results in the formations of diazo radical as well as copper(II) halide, which actually do not participate in the reaction.
Then an N2 molecule is released by the diazo radical to give Aryl radical, which then reacts with copper(II) halide to restore catalyst(copper(I) halide). After all this, the final product obtained is aryl halide.
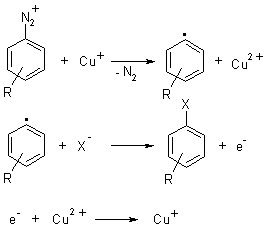
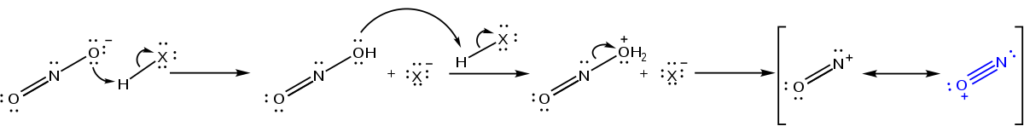
Formation Of The Nitrosonium Ion
For the formation of Nitrosonium ion, we react sodium nitrite with an acid which results in the formation of nitrous acid. This is followed by two protonation steps where one equivalent of water is removed. This generates the Nitrosonium ion.
This ion acts as an electrophile when it is reacted further with an aromatic or heterocyclic amine (for e.g. aniline). Finally, the diazonium salt is formed.
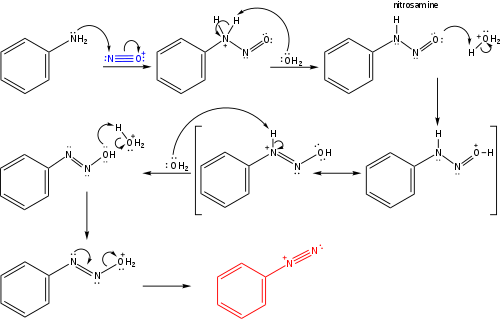
Formation Of The Benzenediazonium Ion
The below image shows the formation of Benzenediazonium in greater detail and makes it easy to understand Sandmeyer reaction.
Applications Of Sandmeyer Reaction
The reaction has multiple synthetic applications as a lot of variations of this reaction have been developed.
Given below are the applications of Sandmeyer reaction:
1. Synthesis of Aryl halides. The solvents used in the process are;
Di-iodomethane: to synthesize Aryl iodides.
Bromoform: to synthesize Aryl bromides.
Chloroform: to synthesize Aryl chlorides.
2. Cyanation: Another use of this reaction is for cyanation which results in the formation of Benzonitriles, which is considered as an important class of organic compound.
Fluanxol, an antipsychotic drug is synthesized by cyanation through this reaction.
3. Trifluoromethylation: This reaction can be used to generate aryl compounds during the process of trifluoromethylation. This process provides unique chemical properties with a wide variety of practical applications.
4. Hydroxylation: This reaction is used in hydroxylation to convert aryl amines to phenols, leading to the formation of an aryl diazonium salt.
FAQs
Copper salts.
Free radical mechanism.
The process of conversion of aromatic amines into its diazonium salt is known as Diazotization.
More Organic Reactions
