Stephen Reaction also known as Stephen Aldehyde Synthesis or Stephen Reduction is named after its inventor Henry Stephen.
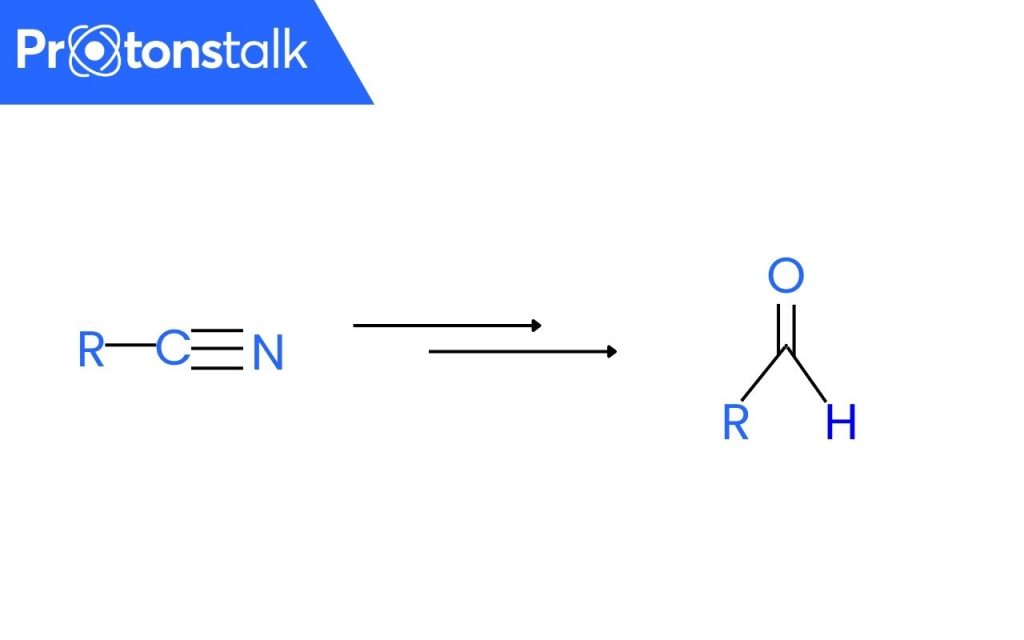
This organic redox reaction involves the preparation of aldehydes(R-CHO) from nitriles(R-CN) using tin(II) chloride(SnCl2), hydrochloric acid(HCl) and quenching the resulting iminium salt(R-CH=NH2+Cl−). (Quenching refers to the rapid cooling with the help of water).
Index
Stephens Reaction Mechanism
Following is the mechanism of Stephens reaction.
- Gaseous hydrogen chloride(HCl) is added to the given nitrile, which reacts to give its corresponding salt.
- A single electron transfer from the tin(II) chloride reduces this salt. On further reduction with HCl, it forms tin (IV) tetrachloride and amine.
- The salt obtained in step 2 precipitates shortly after as aldimine tin chloride.
- The hydrolysis of aldimine tin chloride yields an amide.
- The required aldehyde is formed from this amide. During this ammonium chloride is also formed.
The detailed mechanism is shown in the figure below:

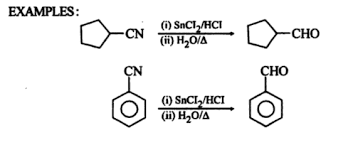
Example
Given below are the examples of Stephens reduction.
Key Points
- Substitutes that can improve the electron density will improve the formation of the aldimine tin chloride.
- Electron withdrawing substituents also promote the formation of amide chloride.
Application
Stephens reduction reaction is used in the preparation of aldehydes by using alkyl cyanides.
FAQs
Stephen reaction is also known as Stephen aldehyde synthesis or Stephen Reduction Reaction. Alkyl nitriles undergo reduction with the help of tin (II) chloride (Sn2Cl) and hydrochloric acid and form an intermediate imine salt which undergoes hydrolysis with water, giving the final result as aldehydes.
The reaction is an organic redox reaction.
More Organic Reactions
