Williamson Reaction or Williamson Ether Synthesis Reaction, is an organic reaction which results in the formation of an ether from an organohalide (organic molecules in which carbons are linked by covalent bonds to halogens) and deprotonated alcohol(alkoxide). The reaction was named after Alexander William Willaimson, who developed it in the year 1850.
Williamson synthesis generally involves nucleophilic displacement of a halide ion or a good leaving group by an alkoxide ion.
The reaction takes place as an SN2 reaction of a primary alkyl halide with an alkoxide ion. This reaction helped to prove the structure of ethers.
Index
Mechanism of Williamson Reaction
The general reaction mechanism is as follows…

- The Williamson ether reaction follows an SN2 bimolecular nucleophilic substitution mechanism.
- So, the nucleophile attacks the electrophile from the backside.
- Here, the alkoxide ion(RO–) acts as a nucleophile, which attacks the electrophilic carbon of the alkyl halide.
- The bond cleavage and the bond formation happens all at once during the nucleophilic attack and ether is formed and halogen separates out.
- This reaction does not favor the formation of bulky ethers due to steric hindrance (prevention due to non bonding interactions) and predominant formation of alkenes instead..
Given below is an example of williamson ether synthesis reaction used to make Dipropyl ether.
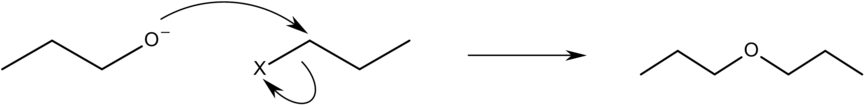
Conditions Required for the Reaction
- As the alkoxide ions are highly reactive, they are usually prepared immediately prior to the reaction.
- In the laboratories, these alkoxide ions are generated by the use of a carbonate base or potassium hydroxide.
- For the industrial synthesis of the alkoxide ions, phase transfer catalysis is used.
- The time taken for the reaction to complete is 1-8 Hrs when conducted at 50 – 100 0 C.
- Solvents used are Acetonitrile and N, N-dimethylformamide .
- The reaction can get a yield of between 50-95% in the lab preparations as the raw materials used undergo side reactions.
- Lab synthesis does not usually require a catalyst but if the alkylating agent is unreactive, iodide salt can be added to improve the rate of reaction which yields in a much more reactive iodide after a halide exchange with the chloride.
- In extreme cases, silver compounds such as silver oxide may be added, which helps in the leaving of the halide group.
Limitations of Williamson Synthesis
- Alkoxides may undergo C-alkylation (form C-C bonds) in addition to expected O-alkylation (form O-C bonds).
- Tertiary alkyl halides or the primary, secondary alkyl halides which are sterically hindered, tend to undergo E2 Elimination in the presence of the alkoxide which also acts as a base.
Applications of Williamson Reaction
- Preparations of ethers in the labs and in industries is done through this process.
- The Williamson synthesis remains the simplest method to prepare both symmetrical and asymmetrical ethers.
- The Williamson reaction is also frequently used to prepare an ether indirectly from two alcohols. One of the alcohols is first converted to a leaving group (usually tosylate), then the two are reacted together.
FAQs
Williamson ether synthesis is an organic reaction which forms an ether from organohalide and alkoxide.This reaction is significant as it has helped to prove the structure of ‘Ethers’.
Ether is a colourless, highly flammable and pleasant smelling liquid. It can be vaporized into gas that reduces pain but keeps the patient conscious.
Williamson ether synthesis is a SN2 reaction.
Williamson synthesis is a process which is used to prepare both basic and mixed Ethers. The alkyl halide is heated to form corresponding ethers with alcoholic sodium or potassium alkoxide.
Example: Sodium ethoxide with chloroethane to form diethyl ether and sodium chloride
[Na]+[C2H5O]− + C2H5Cl → C2H5OC2H5 + [Na]+[Cl]−
More Organic Reactions
