Hell-Volhard Zelinsky Reaction is an organic reaction by which the carboxylic acids are halogenated at the α-carbon atom. This reaction is named after the 3 German-chemists namely: Carl Magnus Von Hell, Jacob Volhard and Nikolay Zelinsky.
The basic reaction is shown below:
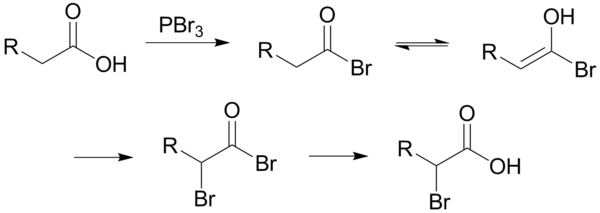
The reaction is initiated by the addition of a catalytic amount of Phosphorus tribromide (PBr3), after which one molar equivalent of Diatomic Bromine (Br2 ) is added. Iodination and fluorination is not possible through this reaction.
Index
Hell-Volhard Zelinsky Reaction Mechanism
This reaction takes place in the absence of a halogen carrier. Halogen carriers take molecules from other compounds to generate electrophiles.
- The Phosphorus tribromide (PBr3) replaces the carboxylic OH with a bromide.
This results in the formation of a Carboxylic acid Bromide. - Next, Tautomerization takes place i.e. acyl bromide/ carboxylic acid bromide tautomerizes to Enol.
- This Enol will readily react with Br2 to brominate at the α-position which results in the formation of α-bromoAcyl bromide.
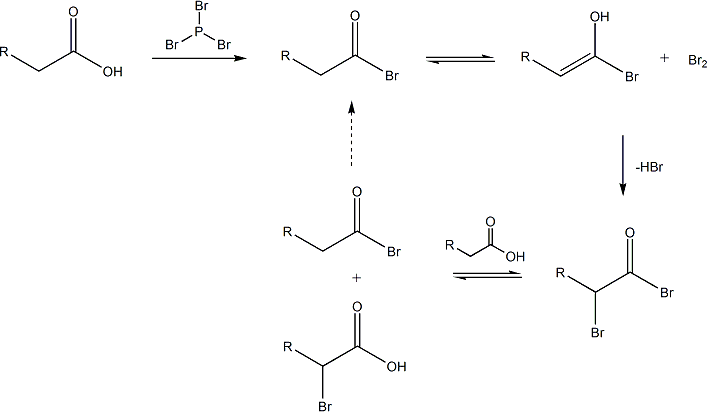
If the aqueous solution is neutral to slightly acidic then, the hydrolysis of the α-bromoAcyl bromide takes place spontaneously, which yields the α-bromo carboxylic acid.
If a little nucleophilic solvent is present then the α-bromo acyl bromide with the carboxylic acids yields the α-bromo carboxylic acid and again generates the acyl bromide intermediate.
Given below is the mechanism of the the exchange between an alkanoyl bromide and a carboxylic acid.
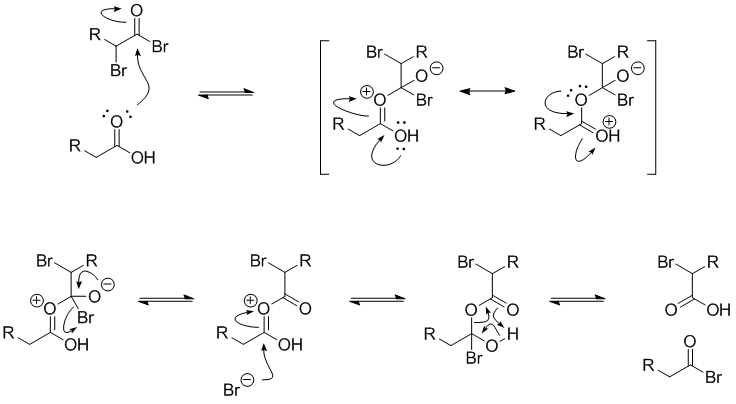
The α-bromoalkanoyl bromide has a strongly electrophilic carbonyl carbon due to the electron-withdrawing effects of the two bromides.
Applications
- Used in the α- bromination of carboxylic acids.
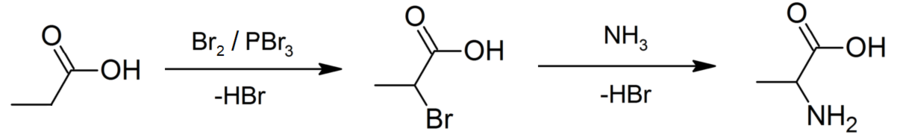
- It is used in the synthesis of amino acids through the displacement of halogens with the help of ammonia (NH3), Given below is an example.
FAQs
Hell-Volhard zelinsky reaction is a process by which the carboxylic acids are halogenated at the α-carbon atom.
The reaction conditions for the Hell Volhard Zelinsky reaction are quite severe- involving reaction temperatures above 373 K and increased reaction time.
More Organic Reactions
