SN2 Reaction is a Nucleophilic Substitution reaction (A class of reactions wherein the electron-rich nucleophile attacks a positively charged electrophile to replace a leaving group) in which two components are involved in the rate-determining step. With simultaneous bond-making and bond-breaking steps, SN2 reactions are bimolecular.
Some of the examples of this reaction mechanism are Swarts Reaction, Finkelstein Reaction and Williamson Ether Synthesis Reaction.
Index
What is SN2 Reaction?
The SN2 reaction is a type of reaction mechanism that breaks one bond and synchronously forms one bond, i.e. it is a single-step reaction. The SN2 reaction is a substitution reaction, the name of which refers to the Hughes-Ingold symbol of the mechanism. “SN” stands for “substitution nucleophilic“, and the “2” says that the rate-determining step is bimolecular.
SN2 Reaction Mechanism
- There is a single transition state, according to the SN2 process, since bond-breaking and bond-making happen simultaneously. Note that the nucleophile must approach from the backside of the carbon (Wherein the front side has the leaving group) for this to occur (so-called backside attack ).
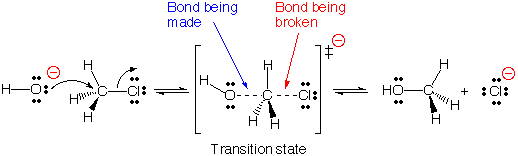
- In an SN2 reaction, there is no intermediate, just a transition state. A transition state does not have a real lifetime, as starting materials transition into products, it is the highest energy point on the reaction coordinate.
Stereochemistry of SN2 Reaction
Theoretically, There are two ways the nucleophile can target the substrate stereo center:
- A frontside attack, where the nucleophile attacks from the same side as the leaving group, resulting in the preservation of the product’s stereochemical configuration.
- A backside attack, where the nucleophile attacks from the opposite side of the carbon-leaving group bond, resulting in the product’s stereochemical structure being reversed.
Since a pure SN2 reaction shows 100% inversion in stereochemical configuration, it is clear that these reactions occur via a backside attack.
The stereocenter configuration is reversed during an SN2 reaction if the halide leaving group is connected to the stereocenter. This is because the nucleophile joins as the departing group (‘backside attack’) from the opposite side of the molecule.
Inhibition by Steric Hindrance
Reactions following SN2 mechanisms are particularly sensitive to steric factors since they are retarded by steric hindrance (crowding) at the site of reaction.
In general, the order of reactivity of alkyl halides in SN2 reactions is:
methyl > 1° > 2°.
The 3° alkyl halides are so crowded that they do not generally react by an SN2 reaction mechanism.
Characteristics of SN2 Reaction
- Bond forming and bond breakage take place in the same step.
- Bimolecular and follows second-order kinetics.
- Tertiary are unreactive.
- Rate of the reaction depends on the concentration of the substrate.
- A polar aprotic solvent is used to enhance the reactivity.
Factors Affecting SN2 Reaction
- A unhindered Substrate(the back of the substrate must be as unhindered as possible).
- A strong Nucleophile.
- A polar aprotic Solvent with low dielectric constant.
- A weaker leaving group.
SN1 Reaction Vs. SN2 Reaction
For SN1 reactions, the step determining the rate is unimolecular, while for SN2 reactions it is bimolecular.
SN1 is a system of two-stage, while SN2 is a process of single stage.
During SN1 reactions, the carbocation forms as an intermediate while it is not formed during SN2 reactions.
Examples
Question 1. Is this SN2 reaction? Explain.
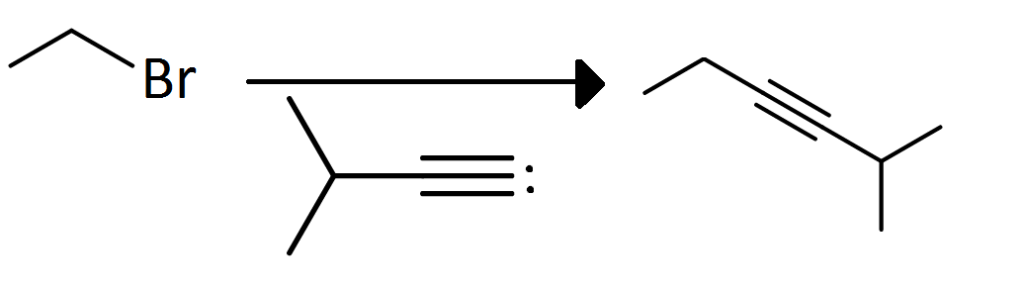
Solution. This is an SN2 reaction. When there is a methyl halide with a strong nucleophile, the nucleophile will force the halide group to leave. Strong nucleophiles dictate SN2 mechanisms.
Question 2. Which of the following would most readily undergo an SN2 mechanism?
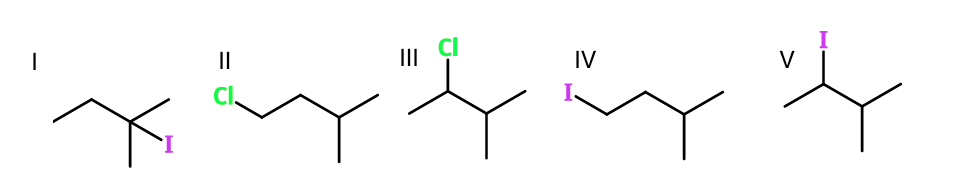
Solution. I– is a better leaving group than Cl– because it is a larger molecule and can distribute the negative charge over a larger area. SN2 works better with better leaving groups and with less-substituted carbons (methyl > primary > secondary > tertiary) so IV undergoes SN2 mechanism most readily.
FAQs
SN2 stands for Substitution Nucleophilic Bimolecular.
SN2 takes place more rapidly. It’s a process that takes place in one step. whereas SN1 is a two-step process in which the first phase, carbocation formation is slow and the second phase, of nucleophile attack is quick. This also depends on the reacting molecules.
The SN2 proceeds through inversion (100%) and gives product with the inverted stereochemistry. Therefore SN2 is stereospecific.

Pingback: SN1 Reaction - Mechanism, Characteristics, Factors | ProtonsTalk
Pingback: Finkelstein Reaction - Mechanism, Applications & Examples | ProtonsTalk
Pingback: Williamson Reaction - Mechanism, Conditions & Applications | ProtonsTalk