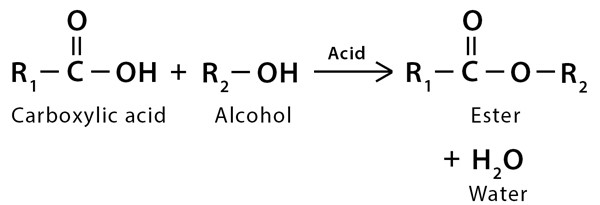
Fischer Esterification is the mechanism of refluxing a carboxylic acid and an alcohol in the presence of an acid catalyst to produce an ester. It is also known as Fischer-Speier Esterification.
The reaction mechanism was first described by Emil Fischer and Arthur Speier in 1895.
Index
Fischer Esterification Mechanism
This mechanism converts carboxylic acids in the presence of excess alcohol and a strong acid catalyst to give an ester as the final product, aligned with water as the byproduct.
Given below is the reaction mechanism.
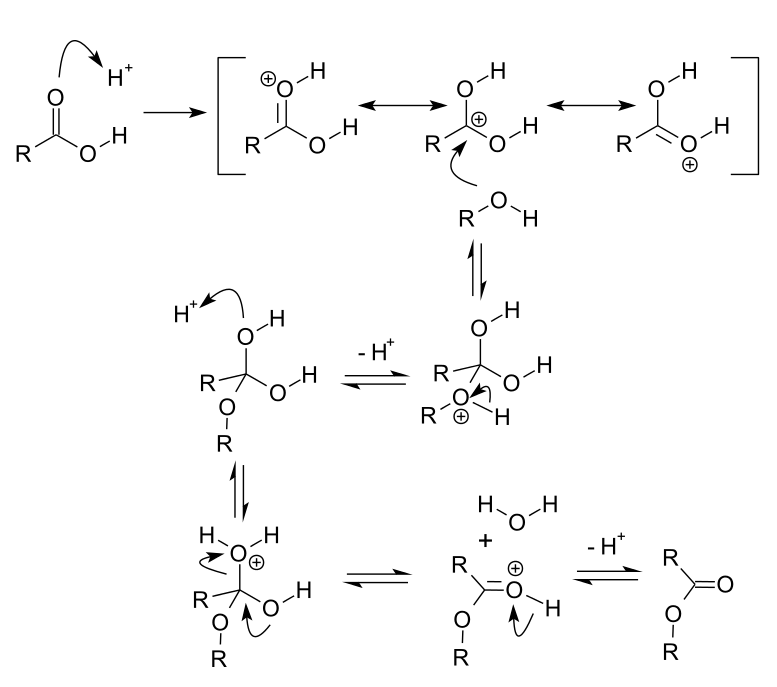
The mechanism has many steps –
- Proton transfer from acid catalyst to carbonyl oxygen which increases the electrophilicity of carbonyl carbon.
- The nucleophilic oxygen atom of the alcohol then attacks the carbonyl carbon.
- Proton transfer from the oxonium ion to the second molecule of alcohol gives an activated complex.
- A new oxonium ion arises from the protonation of one of the hydroxy groups of the activated complex.
- Loss of water from this oxonium ion and subsequent detonation gives the ester (final product).
Advantages and Disadvantages of Mechanism
Advantages
- Relatively simple compared to other esterification mechanisms.
- As compared to other esterifications, the chemicals used and the byproducts released in this mechanism are non-toxic to the environment.
Disadvantages
- As most steps of the mechanism are reversible, the whole process eventually takes a long time.
- This process uses a strong acid. If a weak acid was used, the reaction time would again increase.
- In the presence of strong acids, tertiary alcohols undergo rapid dehydration.
Applications of Fischer Esterification
The mechanism is majorly used to produce esters which then finds it use in a range of synthetic and biological applications. They are for example used as solvents for lacquers, paints, and varnishes.
FAQs
It is essential to use a drying tube in the setup for the mechanism, as the byproduct is water. Water drives the reaction in the backwards direction, which is not desired.
It is the process of converting carboxylic acids into esters, in the presence of excess alcohol and a strong acid.
This can be done by washing the contents with two-third’s of de-ionized water.
More Organic Reactions
