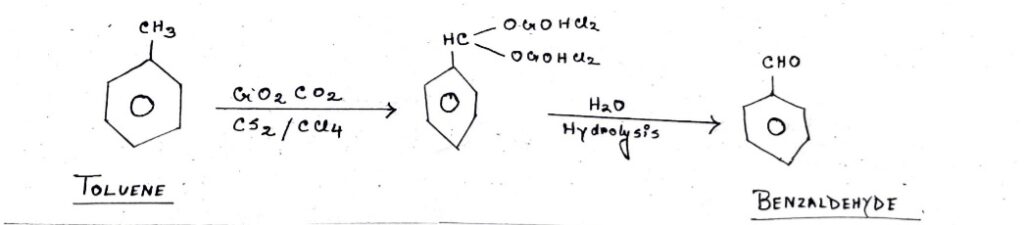
Etard Reaction is the partial oxidation of an aromatic ring with an attached methyl group. For example, converting Toluene (C7H8– A hexagon with a methyl group-CH3) into aldehydes like Benzaldehyde (C7H6O – A hexagon with an aldehyde group-CHO).
Etard reaction is generally broken into two parts. First, where an unstable complex compound is formed and then the final product is formed after reacting with water.
In the case of Toluene forming Benzaldehyde, Toluene reacts with the help of Chromyl Chloride (CrO2Cl2) in the medium of Carbon Disulphide (CS2) and Carbon tetrachloride (CCl4). Toluene forms a complex compound that is C7H6O which is also known as intermediate in this reaction. It again undergoes hydrolysis to achieve our desired product of C7H6O-Benzaldehyde.
Index
History
The name “Etard” was given after French chemist Alexandre Léon Etard (1852 – 1910) who devoted most of his life in Wurtz Laboratory. He discovered the partial oxidation of aromatic rings with methyl groups using Etard reagent or commonly known as chromyl chloride(CrO2Cl2).

Mechanism of Etard Reaction
Let’s again take the example of Toluene forming into Benzaldehyde to understand the mechanism of Etard reaction.
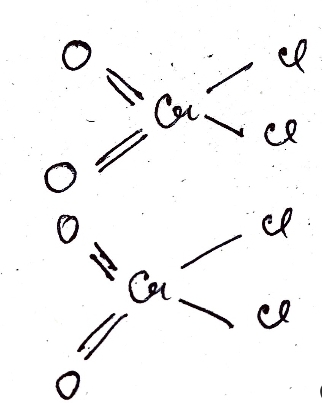
- Toluene reacts with CrO2Cl2(Chromyl Chloride) and that causes four homolytic cleavages. (covalent bond breaking in such a way so that each part gets equal bits of the total electrons contained in the bond.)
- Two carbon hydrogen (C-H) bonds of methyl group on Toluene undergo homolytic cleavage.
- One chromium oxygen (C-O) single bonds undergo homolytic cleavage. (This cleavage occurs in two Chromyl Chloride molecules.) (As only a single chromium oxygen bond breaks, oxygen is still attached to Cr.)
- The hydrogens which break from Toluene comes and bond with the oxygens of two Chromyl Chloride molecules which also went through a cleavage forming two OCr(OH)Cl2.
- The two OCr(OH)Cl2 molecules gets attached to the carbon of the methyl group of Toluene through the second oxygen. (It basically occupies the position from where hydrogens got cleaved.)
- It forms an unstable complex compound that is intermediate in the Etard reaction.
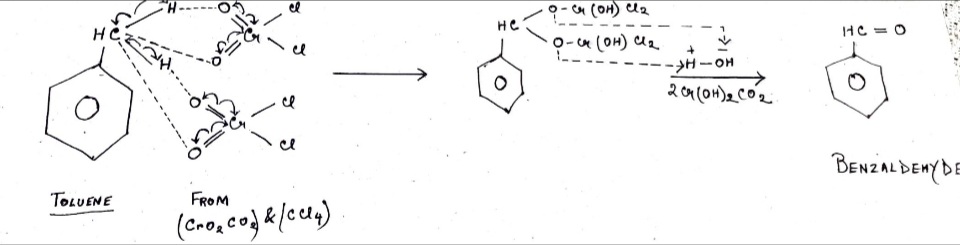
- We perform hydrolysis on the intermediate. Due to the instability of the formed compound. There will be a moderate electron exchange between the two oxygens with the Carbon of the Toluene part.
- The Oxygen in the first OCr(OH)Cl2 pulls electrons from carbon, breaks C-O bond and gets attached to H+ of water forming OCr(OH)2Cl2.
- One valence of carbon is free. So, the oxygen in second OCr(OH)2Cl2 will pull its electrons from the O-Cr bond, break the O-Cr bond, and form a double bond with Carbon. The remaining positive Cr(OH)Cl2 bonds with OH- of water again forming OCr(OH)2Cl2.
- Two OCr(OH)Cl2 forms two OCr(OH)2Cl2 will get separated leaving behind the product which is Benzaldehyde.
Limitations of Etard Reaction
Reacting with aromatic rings other than toluene to form different required aldehydes is difficult in this reaction. Even if we use strong oxidizing agents, the aromatic rings will form carboxylic acids instead of aldehydes.
Applications of Etard Reaction
- The Benzaldehyde (Product of this reaction) is used as the base for the formation of perfumes
- Benzaldehyde is also used as a substitute for the almond flavor in food companies
- Benzaldehyde is used in the synthesis of phentermine.
So, that was all about another named organic reaction, Etard Reaction
FAQs
The reagent used is Chromyl Chloride(CrO2Cl2). It’s a mild oxidizing agent also known as Etard reagent.
Toluene can be converted to benzoic acid by undergoing full oxidation with the help of a strong oxidizing agent like KMnO4.
We can convert Toluene into Benzaldehyde by reacting Toluene with Chromyl Chloride in the medium of CS2 and CCl4 and then hydrolyzing the intermediate product.
Etard reaction is the partial oxidation of an aromatic ring with an attached methyl group to form desired aldehydes.
