Diethyl Ether is an organic compound, which is simply called ether and belongs to the ether class. This organic compound is a structural isomer of butanol.
Index
History
This compound was synthesized by a German physicist, botanist, and pharmacologist Valerius Cordus, in 1540. It was also known as “sweet oil of vitriol”(oleum dulce vitrioli), as the name reflects the fact that it is obtained by distilling a mixture of ethanol and sulfuric acid. The name ether was given to the compound by August Sigmund Frobenius in 1729.
Structure & Formula
Diethyl ether has the chemical formula (C2H5)2O and the compound’s structure is represented below.
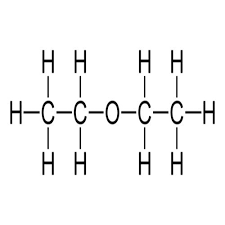
Physical Properties
| Odor | Dry, rum-like sweetish odor |
| Appearance | Colorless liquid |
| Melting Point | -116.3℃ |
| Boiling Point | 34.6℃ |
| Density | 0.7134 g/cm3 |
| Solubility | Soluble in water |
Chemical Properties
- Ether is a highly flammable liquid and undergoes combustion to form carbon dioxide and water.
C2H5OC2H5 + 6O2 → 4CO2 + 5H2O
- Ether reacts with halogens such as chlorine and bromine to form halo substituted ether. This is a substitution reaction in the absence of sunlight.
C2H5OC2H5 + Cl2 → C2H4(Cl)OC2H4(Cl)
Preparation
- This organic compound can be obtained by heating ethyl iodide with dry silver oxide.
- By heating ethyl alcohol with the compound alumina (Al2O3) at 250°C, we can obtain diethyl ether.
C2H5OH + HOC2H5 + Al2O3/250°C→ C2H5 — O — C2H5 + H2O - It can be prepared by Williamson Synthesis Method wherein sodium or potassium ethoxide is heated with chloro, bromo or iodo ethane.
C2H5ONa + C2H5I → C2H5 — O — C2H5 + NaI
Applications
- Diethyl ether is used as a starting fluid, in combination with petroleum distillates for gasoline and diesel engines.
- This compound was also used as a general anesthetic in surgeries, but was discontinued due to its undesirable side effects, post-anesthetic vomiting.
- It is used to treat hiccups through instillation into nasal cavity.
- It is also used as a common laboratory solvent.
Hazards
This compound is extremely flammable and has the ability to form explosive air mixtures.
It is harmful if swallowed. It may also cause drowsiness and dizziness upon exposure.
FAQs
It is recommended to be highly cautious and careful while working with diethyl ether as the compound is extremely flammable and may cause unconsciousness when high concentrations inhaled.
Diethyl ether is produced by mixing ethyl alcohol with a strong acid, like sulfuric acid. This mixture is heated to about 140℃ to obtain diethyl ether.
C2H5OH + H2SO4 → C2H5HSO4 + H2O
C2H5HSO4 + C2H5OH → C2H5OC2H5 + H2SO4
It is used as a solvent for fats, oils, dyes, gums, raw rubber, smokeless powder, perfumes, etc.
