The Rosenmund Reduction reaction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction was named after Karl Wilhelm Rosenmund who discovered it in 1918.
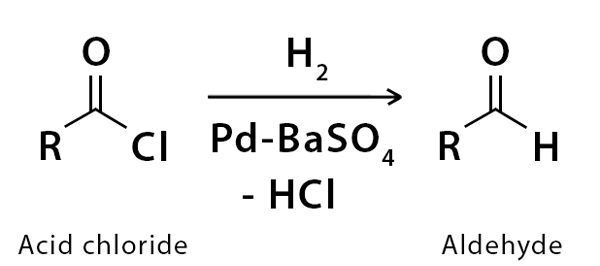
In this organic reaction, the molecular hydrogen reacts with the acyl chloride in the presence of catalyst–palladium on barium sulfate which is also known as Rosenmund catalyst. Rosenmund catalyst is made by reducing palladium(II) chloride solution in the presence of BaSO4
Index
Rosenmund Reduction Mechanism
Hydrogen gas in presence of Rosenmunds catalyst is passed through acyl chloride, resulting in the formation of an aldehyde and hydrochloric acid.
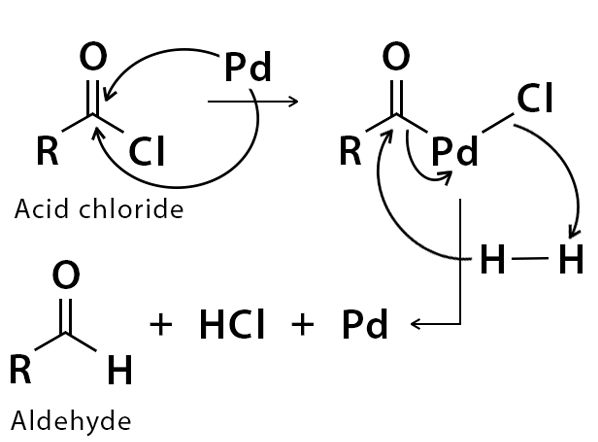
The resulting aldehyde formed can undergo further reaction with the palladium over barium sulfate and can lead to a formation of primary alcohol which can even further react with remaining acyl chloride forming an ester.
This further reaction needs to be stopped or reduced which is done by poisoning the catalyst.
Barium sulfate has a low surface area which reduces the activity of the palladium, preventing over-reduction. But, certain reactive acyl chlorides must be stopped from further reduction by the addition of poison.
Poisoning is a process by which we can partially or totally deactivate a catalyst using a chemical compound. In this case, the poison used is Thioquinanthrene.
Applications of Rosenmund Reaction
Coming to the applications of Rosenmund Reaction
- The Rosenmund reaction method is used to prepare several aldehydes like alkyl or aryl aldehydes.
- It is also used for the production of saturated fatty aldehydes.
Limitations of Rosenmund Reaction
Many aldehydes can be prepared by this reduction mechanism but formaldehyde cannot be prepared by this method, as formyl chloride is unstable at room temperatures.
FAQs
The rosenmund reaction is catalyzed by palladium on barium sulfate. Barium sulfate reduces the activity of palladium due to its low surface area meaning it decreases the reducing power of palladium in order to prevent over-reduction of the acyl chlorides.
The reduction is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst.
Formaldehyde , because formyl chloride is unstable at room temperature.
Thioquinanthrene is the poison used in the reaction. It basically prevents the further reaction of aldehyde which forms alcohols and esters.
