The 18 Electron Rule is similar to the octet and duplet rules, as it is used to predict stability.
There are two 18 electron rules to be precise; one is applicable to the transition elements and another is for organometallic compounds. Though these 18 electron rules are different in their areas of application, their underlying theory and purpose are almost the same.
Index
History
This rule was first proposed by the American chemist, Irving Langmuir. He proposed this rule in order to extend the Lewis static-atom model further than Argon in the periodic table.

Later a new method to predict the stabilities of organometallic compounds was proposed by Nevil Sidgwick, called the Effective Atomic Number rule (EAN).
Due to this new EAN rule Irving’s 18 electron rule was less used by other scientists for a while. But later many reverted to the 18 electron rule as the EAN rule had different effective atomic numbers for every period of the transition group for a compound to be stable. Whereas the 18 electron rule is more like octet rule which can be applied to the whole group with the same numerical value.
18 Electron Rule Explained
The 18 electron rule states that,
For a transition element to be stable it needs to have 18 electrons in its valence shell so that they have the configuration of an inert gas, and that they form chemical bonds in order to attain this state.
This rule was proposed in order to explain the stability of transition metals and the organometallic compounds which they form.
The law is based on the fact that, in the valence shell of transition elements the total number of electrons is 2 s electrons, 6 p electrons and 10 d electrons making a total of 18 electrons.
Though this rule is often called a Chemical Rule of Thumb, transition elements often do not follow this rule.
Due to this fact, a condition called duodectet rule arises. Where the stability is determined by 12 electrons in the valence shell rather than 18 electrons. This condition arises due to the high energy and more diffuse p-orbitals which take part in a reaction only under specific conditions.
Effectively, the 12 and 18 electron rules describe the lower and upper bounds of valence electrons for a stable organometallic compound.
Though this rule just gives a bound on the maximum valence electrons of a transition element, it is also used to predict the stability of an organometallic compound and reactivity of a transition element.
Elements with valence electrons close to 18 are more reactive, so that they can achieve 18 electrons. The compounds with 18 electrons around their central metal atom are often relatively more stable than the others.
Limitations of 18 Electron Rule
There are many elements and compounds which do not follow the 18 electron rule as mentioned above.
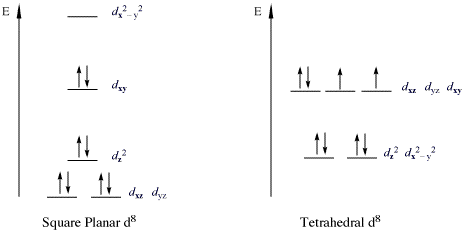
- 16 Electron Compounds: The main group which do not follow this rule are those compounds which are called 16 electron compounds. There are two types of 16 electron compounds, high spin which are octahedral and low spin which are square planar. The low spin 16 electron compounds are relatively more stable due to their low energy bonding orbitals, due to which they do not follow this rule.
- Bulky Ligands: The other group of compounds which commonly violate the rule are those with bulky ligands. The bulky ligands present in the compound block the participation of all the orbitals in bonding by hindering the approach of other ligands for bonding.
- High Spin Compounds: High spin compounds often do not have enough vacant orbitals for accepting enough electron pairs from ligands to gain 18 electrons in their valence shells.
- Higher Electron Compounds: There are compounds with electrons more than 18 in their valence shell after bonding. This usually happens when an element tries to balance its positive charge by gaining electrons from ligands, and sometimes it ends up with more than 18 electrons. This also happens when chelating(forming of rings) stabilises the molecule more than the rule.
- ?-donating Ligands: The ligands with lone-pairs on the coordinating atom stabilise unsaturated compounds. These ligands are called ?-donating ligands and hence the compound does not follow the 18 electron rule.
- Other Block Elements: This rule is only applicable to transition elements and cannot be applied to find the stability and reactivity of elements of s- and p-blocks, as they do not have d-orbital in their valence shell to gain 18 electrons. It is not applicable even to f-block elements.
- Multiple Metal Atoms: When there are more than 6 metal atoms in an organometallic compound the 18 electron rule is not obeyed by it.
Applications of 18 Electron Rule
- Predicts the stability of various organometallic compounds.
- Predicts the reactivity of transition elements.
- Used to predict and refine the formulas of various complex compounds, especially organometallic compounds.
- Used to predict the existence of metal-metal bonds in complexes.
- Total number of metal-metal bonds in an element(N),
\(N = \frac{(n*18)-A}{2}\)
Here,
\(n\) is the number of metal atoms in the complex.
\(A\) is the total valence electrons (TVE) of the complex. (which is sum of valence electrons of the metal and the electrons gained from each ligand)
Examples
Question 1. Give examples of compounds that follow the 18 electron rule.
Answer. [Co(NH3)6]Cl3 , Mo(CO)6 and [Fe(CN)6]4-.
Question 2. Find the number of metal-metal bonds in the compounds:
- Fe3(CO)12
- Co4(CO)12
Answer.
1. Fe3(CO)12
Here, the number of metal atoms(\(n\)) = 3
Total valence electrons(\(A\)) = 48
Then, \(N = \frac{(n*18)-A}{2} = \frac{(3*18)-48}{2} = 3\)
2. Co4(CO)12
Here, the number of metal atoms(\(n\)) = 4
Total valence electrons(\(A\)) = 60
Then, \(N = \frac{(n*18)-A}{2} = \frac{(4*18)-60}{2} = 6\)
FAQs
The 18 electron rule is a rule which the transition elements follow when forming bonds and also when forming complex compounds. They try to attain the inert gas configuration in their valence shell by forming bonds and complexes, hence following the 18 electron rule.
No, this rule can only be applied to transition elements and their complexes because they have s-, p- and d-orbitals, whereas p-block elements only have s- and p-orbitals in their valence shells.
The rule fails due to various reasons:
a) bulky ligands
b) high spin compounds
c) multiple metal atoms
d) other block elements
e) compounds with more than 18 electrons due to stability reasons
The 18 electron rule and EAN rule are very similar but they are not the same as the EAN value of stable complexes changes with period(i.e. different for each period of the periodic table), whereas that value for the 18 electron rule is the same for the whole block of transition elements.
