Carboxylic acid is basically an organic acid containing a carboxyl functional group (C(=O)OH) attached to an R-group. The general formula is R-COOH or R-CO2H. R refers to the alkyl, alkenyl, aryl, or another group.
These acids are a highly important functional group that presents the C=O bond.
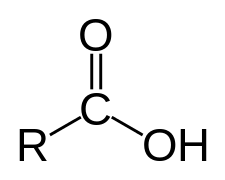
Common examples of carboxylic acids are acetic acid (CH3CO2H), formic acid (HCOOH), benzoic acid (C6H5CO2H), oxalic acid (HO2CCO2H), etc.
Index
Nomenclature of Carboxylic Acid
Carboxylic acid often has the suffix -ic acid in their trivial names which is useful in identifying them. Their IUPAC names end with -oic acid. The carboxylate anion (R-COO– or RCO2–) of these acids usually has the suffix -ate.
IUPAC Nomenclature Guidelines
- The suffix “e” is replaced by “oic acid” in the corresponding alkane.
- The carboxylic carbon is numbered one when only one carboxyl group is present in the aliphatic chain.
- If more than one carboxyl group is present then the total number of carbon atoms is counted and the number of carboxyl groups is represented as “di-”, “tri-”, etc.
- Concatenating these prefixes and suffixes to the parent chain gives the names of the corresponding carboxylic acids.
- The name “carboxylic acid” or “carboxy” can be assigned to a carboxyl substituent on a carbon chain.
Properties of Carboxylic Acid
Now, let’s move on to discussion of properties of carboxylic acids which implies physical properties and chemical properties.
Physical Properties
- Smaller carboxylic acids are soluble in water compared to bigger carboxylic acids due to the increasing hydrophobic nature of the alkyl chain. Bigger carboxylic acids are soluble in less polar solvents such as ethers and alcohols.
- These acids tend to have higher boiling points than water, because of their greater surface areas and their tendency to form stabilized dimers through hydrogen bonds.
- The carboxylic acids are weak acids, i.e., they only partially dissociate into H3O+ cations and RCOO- anions in a neutral aqueous solution.
- Carboxylic acids have the ability to donate protons and are therefore Bronsted-Lowry acids.
- These acids have strong odours. Esters of these acids have pleasant odours.
Chemical Properties
- These acids are involved in reactions such as acid dissociation and solvolytic reactions.
- They also undergo decarboxylation in which the R-C bond is broken in such a way that CO2 is lost and R-H is formed.
- These acids yield esters, upon reaction with alcohols.
- These acids can be converted into amines using the Schmidt reaction.
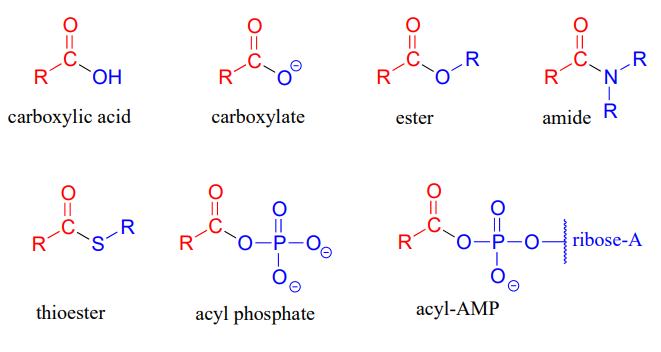
Derivatives
Below listed are the different carboxylic acid derivatives.
- Carboxylate
- Ester
- Amide
- Thioester
- Acyl phosphate
- Acyl-AMP
- Acid Chloride
- Acid anhydride
The structures of these derivatives are displayed below (Source).
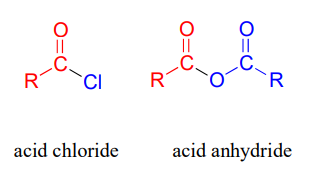
Carboxylic acid chlorides and anhydrides are not usually found in biomolecules but are useful intermediates in laboratory synthesis.
Occurrence and Synthesis
Following are the laboratory methods used to synthesize carboxylic acids:
- Oxidation of primary alcohols or aldehydes with strong oxidants such as potassium dichromate, Jones reagent, etc.
- Hydrolysis of nitriles, esters, or amides with acid or base catalysis also produces these acids.
- Carbonation of a Grignard reagent and organolithium reagents:
RLi + CO2 → RCO2Li
RCO2Li + HCL → RCO2H +LiCl - Halogenation followed by hydrolysis of methyl ketones in the haloform reaction synthesizes carboxylic acids.
Applications of Carboxylic Acid
- Carboxylic acids are used in the production of polymers, pharmaceuticals, solvents, etc.
- They are also used to produce food additives.
- Carboxylate salts are used as soaps.
- Acetic acid, an example of carboxylic acid, is used as a coagulant in the manufacture of rubber.
- It also find applications in the textile and leather industries.
FAQs
A carboxylic acid is an organic compound containing a carboxyl functional group (C(=O)OH) attached to an R-group.
Formic acid, methanoic acid, oxalic acid, etc., are examples of carboxylic acids.
