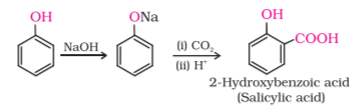
Kolbe’s Reaction or Kolbe-Schmitt Reaction is a Carboxylation reaction. The final main product is an aromatic hydroxy acid, popularly known as Salicylic Acid.
Carboxylation is the reaction in which a substance is treated with carbon dioxide(CO2) to produce carboxylic acid group(R-COOH).
This reaction was discovered by and named after Hermann Kolbe and Rudolf Schmitt. Hermann Kolbe contributed greatly to the birth of Modern Organic Chemistry.

Index
Kolbe’s Reaction Explained
Phenol(C6H5OH) is reacted with Sodium Hydroxide(NaOH) to form Sodium Phenoxide(C6H5ONa), which is more reactive towards Electrophilic Aromatic Substitution. Then Sodium Phenoxide is treated with CO2 in the presence of H+(any acid like H2SO4) to form Salicylic Acid/Ortho-Hydroxybenzoic Acid.
Kolbe’s Reaction Mechanism
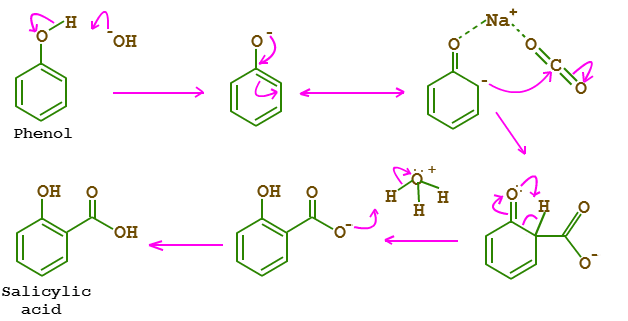
Step 1: Phenol reacts with sodium hydroxide to form phenoxide ion.
Step 2: The negative phenoxide ion shifts to ortho position and forms a transition state with Na+ and CO2 simultaneously.
Step 3: The -ve ion attacks on Carbon of CO2 and proton shift takes place from ortho position to Oxygen attached to the benzene ring.
Step 4: At the same time, as of proton shift, H+/H3O+(from any mineral acid like H2SO4) attacks on carboxylate ion.
Therefore, Salicylic acid/Ortho-hydroxy Benzoic acid is formed with H2O as a by-product.
Example
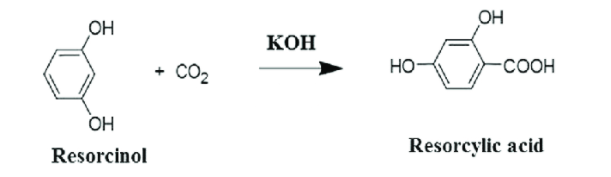
The following reaction also follow Kolbe’s reaction procedure
Resorcinol reacts with CO2 in presence of KOH to form Resorcylic acid (or) 2,4-dihydroxy benzoic acid.
Applications
- Salicylic acid undergoes Esterification (here, reacts with acetic anhydride) to form Aspirin. Aspirin is used as a painkiller in medicine.
- Salicylic acid is used to produce 3-hydroxy 2-naphthoic acid, which is used in forming many dyes and pigments.
- Salicylic acid is also used in the formation of 4-hydroxybenzoic acid. It is an important chemical used for the production of Personal care or Cosmetic products.
FAQs
It is similar to the reaction of Grignard reagent with CO2.
Kolbe’s reaction and Kolbe-Schmitt reaction are the same. Whereas, Kolbe’s Electrolysis is the reverse of Kolbe’s reaction, which involves Decarboxylation of Carboxylate salts (eg. Potassium Carboxylate) into Alkanes.
Sulfuric acid (H2SO4) is mostly used as a reagent in this reaction.
Phenoxide ion is more reactive which can easily donate electrons to the benzene ring than the -OH group of phenol.
More Organic Reactions
