A critical point or critical state is the endpoint of the phase equilibrium curve. It is the highest temperature and pressure at which a pure substance can exist in vapour/liquid equilibrium.
For example – the liquid-vapour critical point is the endpoint of the pressure-temperature curve condition under which a liquid and its vapour coexist.
A substance at a temperature above the critical temperature can no longer be liquified, regardless of the amount of pressure applied to it.
Index
History
The critical state of a liquid was first discovered by Charles Cagniard de la Tour, French physicist in 1822.

He observed that carbon dioxide (CO2) could be liquified at a temperature of 31°C when pressure of 73 atm was applied, but it would not get liquified even at higher temperatures, when pressures above 3000 atm was applied.
He discovered supercritical fluids while conducting experiments involving the discontinuities of the sound of a flint ball in a sealed cannon barrel filled with various fluids at various temperatures.
The maximum temperature at which a substance exists in the liquid phase was named “Critical Temperature” by Dmitri Mendeleev in 1860.
Critical Point Explained
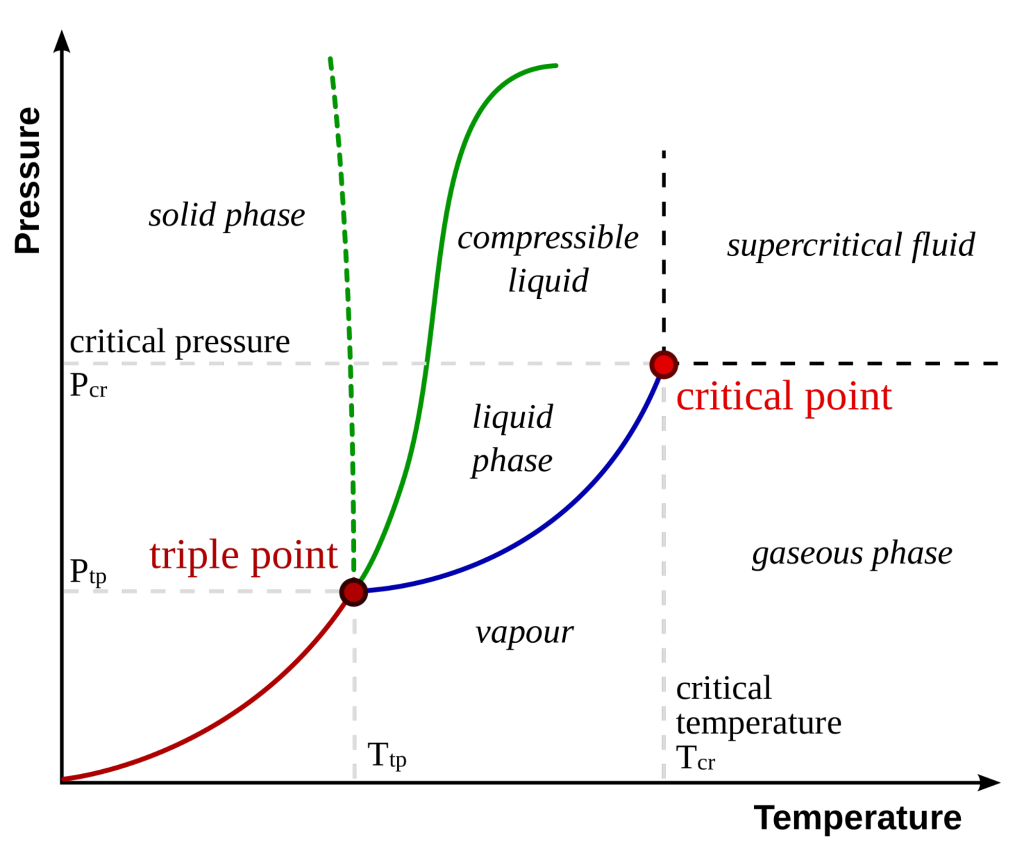
The graph here shows the Pressure vs. Temperature diagram of a pure substance. The commonly known phases (solid, liquid, and vapour) are separated by the phase boundaries.
i.e. a pressure-temperature combination where the two phases can coexist.
However, at the triple point, all three phases can coexist.
The liquid-vapour boundary terminates in an endpoint at some critical pressure (Pc) and critical temperature (Tc). This is the critical state.
The critical state in water occurs at 647.096 K (373.946°C; 705.103°F) and 22.064 mega-pascals (3,200.1 psi; 217.75 atm).
Applications of Critical Point
To purify a substance from a mixture, which has a very high critical state, pressure and temperature are externally regulated to get the desired the purified substance separated.
Questions
Question. Define the Critical Temperature of a gas. Give its expression.
Solution. The critical temperature (Tc) is that temperature after which gases cannot be liquified further. Different gases have a different critical temperature. Example CO2 has 31°C as its critical temperature.
The equation can be derived from Van-Der Waals equation
\((P + \frac{a}{V^2})(V – b) = RT\)and by little rearrangement we can get,
\(T_c = \frac{8a}{27Rb}\)
here, \(R\) = Gas Constant
FAQs
A critical point(or critical state) is the endpoint of a phase equilibrium curve, the conditions under which a liquid and its vapour can coexist is designated by the endpoint of the pressure–temperature curve.
At the triple point, all three phases (solid, liquid, and gas) are in equilibrium and co-exists.
The critical point is the highest temperature and pressure at which a pure substance can exist in vapour/liquid equilibrium.
A supercritical fluid(SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid.
At very high pressures, an SCF can be compressed into a solid because the melting curve extends to the right of the critical point in the Pressure-Temperature phase diagram.
