Hoffmann Bromamide reaction is an organic reaction in which a primary amide is converted to a primary amine. After conversion, the primary amine has one less carbon atom.
The reaction was discovered by August Wilhelm von Hofmann, and hence is named after him.
Index
More about the Reaction
The reaction is also known as Hoffmann Bromamide Rearrangement (or) Hoffmann Bromamide Degradation.
In this reaction, an amide is treated with bromine in an aqueous or ethanolic solution of sodium hydroxide which leads to the degradation of amide and the formation of primary amine.
The basic reaction is shown below:
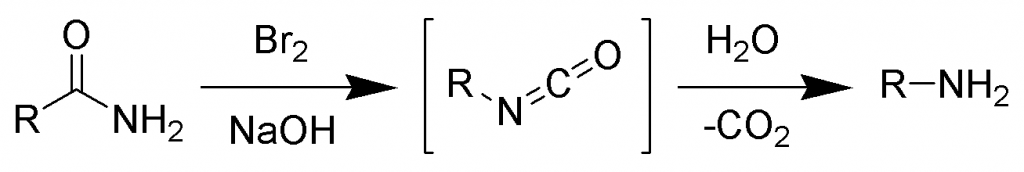
Hoffmann Bromamide Reaction Mechanism
The reaction can be explained in the following steps:
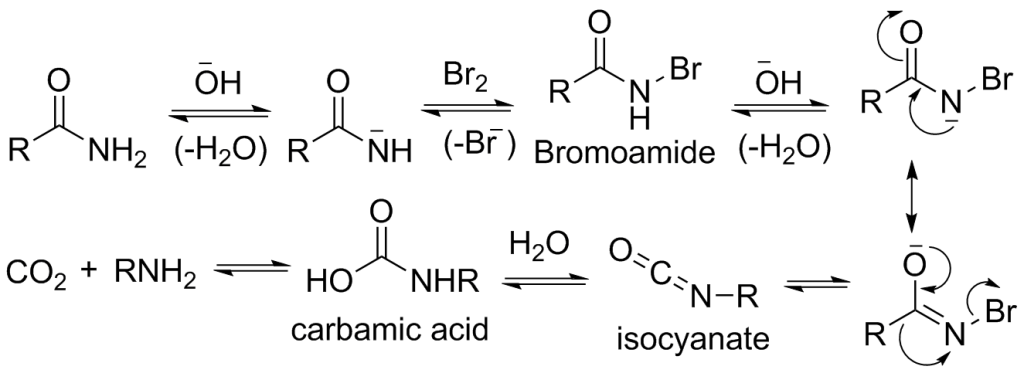
- The primary amide reacts with sodium hydroxide (NaOH). The primary amide is attacked by the hydroxide ion (OH-) of the NaOH, hence deprotonation of the primary amide takes place which leads to the formation of an intermediate Isocyanate (primary amide ion).
- When bromine reacts with the primary amide ion, it results in the formation of R-CO-NHBr (Bromamide) and elimination of Br.
- Another molecule of sodium hydroxide (NaOH) reacts with R-CO-NHBr, which results in the formation of R-CO-NBr.
- The bromoamide anion rearranges as the R group attached to the carbonyl carbon migrates to nitrogen at the same time the bromide ion leaves, giving an isocyanate.
- Now, water molecules are added to the isocyanate, to form carbamic acid (urethane). This is an example of a nucleophilic addition reaction.
- At last, the carbamic acid loses CO2, which results in the formation of primary amine.
Applications of Hoffmann Bromamide Reaction
- The reaction is used in the preparation of anthranilic acid from Phthalimide.
- It is used to produce primary Aromatic and aliphatic amines.
- Also used in the preparation of Aniline.
- Nicotinamide is converted to 3-AminoPyridine.
Example
Here, Hofmann degradation is shown by Acetamide, where it is converted to methanamine. The reaction is shown below.
CH3-CO-NH2 (Acetamide) + Br2 + 4NaOH → CH3-NH2 (Methanamine) + Na2CO3 + 2NaBr + 2H2O
FAQs
It is basically a reaction of primary amide with a halogen (chlorine or bromine) in a Strongly basic aqueous medium (NaOH). The primary amide is converted to primary amine.
It is a nucleophilic addition reaction.
More Organic Reactions
