Ammonium persulfate (NH4)2S2O8 is a white crystalline inorganic compound. It is a white powder-like salt and is highly soluble in water. It has the chemical formula (NH4)2S2O8.
Hugh Marshall prepared this compound for the first time.
Index
Structure and Chemical Formula
Ammonium persulfate is an ionic compound with a +1 charge on the cation and a -2 charge on the anion. Its chemical formula is (NH4)2S2O8 and its structure is as follows.
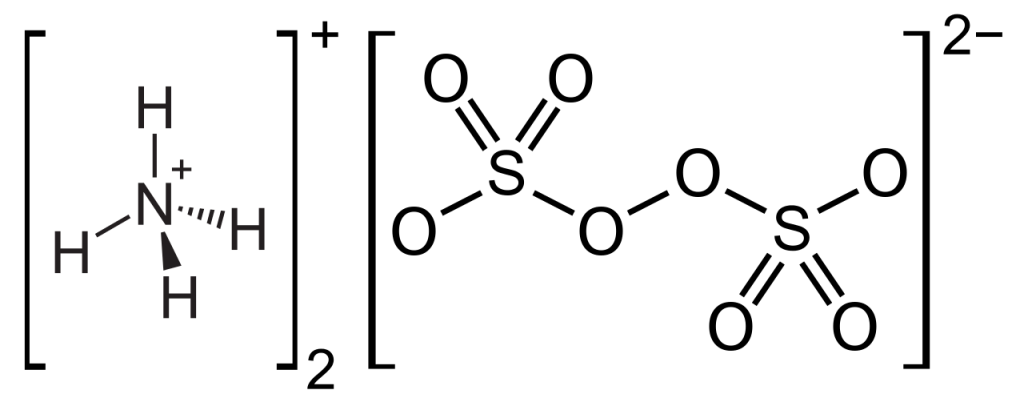
The value of ammonium persulfate molar mass is 228.18 g/mol.
Physical Properties
| Appearance | White, Crystalline Solid |
| Molar Mass | 228.18 g/mol |
| Density | 1.98 g/cm³ |
| Melting Point | 120°C |
| Solubility | 80 g/100 mL (25 °C) in water. Moderately soluble in MeOH |
| Heat Capacity | 529.7 J/mol/K |
| Odour | Mild, Unpleasant Odour |
Chemical Properties
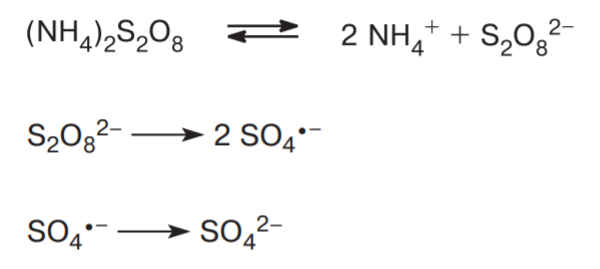
- The white crystalline compound decomposes in an aqueous medium to form ammonium ions and peroxydisulfate oxyanions. This further decomposes into sulfate ions. Therefore the decomposition of the compound can result in the formation of sulfuric acid fumes.
- Oxidation of alkynes using ammonium persulfate and diphenyl diselenide as catalyst in aqueous media results in 1,2 dicarbonyl derivatives or hemiacetals (starting from terminal alkynes).
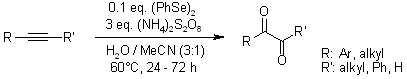
- It is a strong and stable oxidising agent.
Production
Ammonium Persulfate can be produced via the electrolysis of ammonium sulfate (NH4)2SO4 and sulfuric acid H2SO4 followed by crystallisation of the electrolytic solution.
The sulfuric acid is electrolysed to hydrogen sulfate ions HSO4⁻ at the anode. 2 hydrogen sulfate ions form peroxydisulfate acid H2S2O8, which reacts with the ammonium sulfate to form ammonium persulfate and sulfuric acid.
H2SO4 ↔ H⁺ + HSO4⁻
Anode Reaction:
2 HSO4⁻ ─ 2e⁻ → H2S2O8
Cathode Reaction:
2 H⁺ + 2e⁻ → H2 ↑
Reaction of Peroxydisulfate Acid with Ammonium Sulfate:
(NH4)2SO4 + H2S2O8 → (NH4)2S2O8 + H2SO4
The remaining solution is crystallised in order to extract the the required compound (NH4)2S2O8.
Applications of Ammonium Persulfate
Some of the several applications of this inorganic compound includes:
- Preservative in food products
- Ingredient in hair bleaches
- Used in PCBs (printed circuit boards)
- Used as a general oxidising agent
- Also used as an initiator in several polymerisation reactions.
Ammonium Persulfate Hazards
(NH4)2S2O8 is an irritant. Inhaling dust containing the compound can irritate the lungs, and in severe cases can also cause asthma.
Coming in contact with this compound can irritate eyes and cause skin allergies. High exposure to the compound can lead to fluid build-up in the lungs or “pulmonary edema”, in which case immediate medical supervision is required.
FAQs
Its chemical formula is (NH4)2S2O8.
Its molar mass is 228.18 grams per mole.
More on Inorganic Compounds
