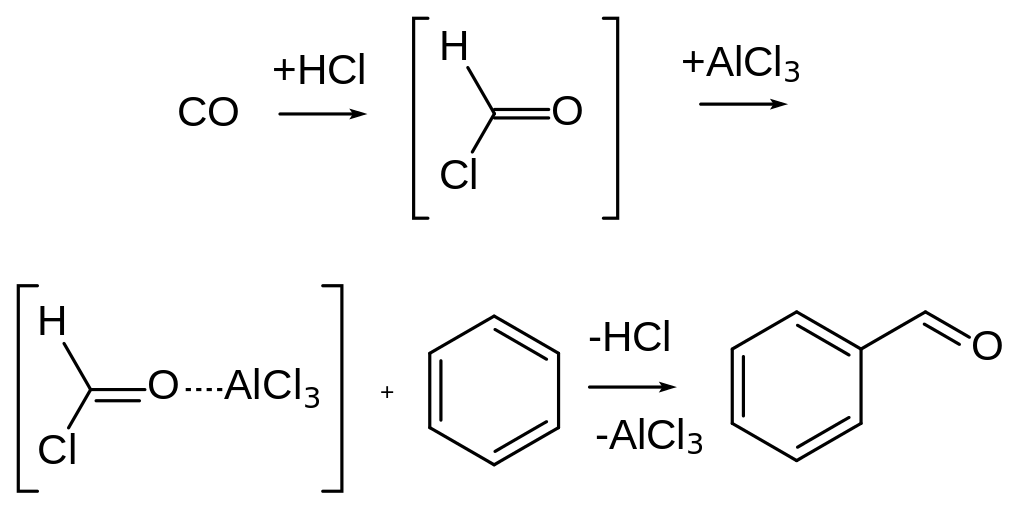
Gattermann Koch Reaction involves formylation (addition of Aldehyde group) of an aromatic compound with the help of formyl chloride (produced by Carbon Monoxide and HCl) and Lewis Catalysts.
The reaction is performed either under pressure or using copper(I) chloride, which aids the formation of formyl chloride (HCOCl).
Index
History
The reaction was developed by L Gattermann and J.A. Koch in 1897. They were successful in the addition of an aldehyde group to toluene using formyl chloride (HCOCl) and Lewis catalysts (e.g., AlCl3, FeCl3, etc.).

Gattermann Koch Reaction Mechanism
Now, let’s discuss the Gattermann Koch reaction mechanism, which involves three major steps discussed below.
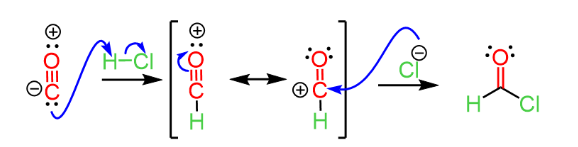
Step-1: Formation of Formyl Chloride
Carbon monoxide attacks HCl molecules due to its nucleophilic nature. A reaction intermediate is formed, which has two resonance structures. Cl– can’t attack the oxygen atom because of the huge size of the oxygen atom. The electrophilic Cl– ion attacks the carbon of this structure. The Final result is Formyl Chloride (HOCl).
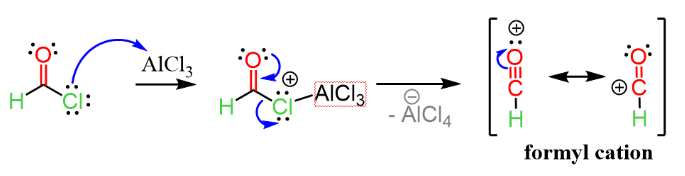
Step-2: Interaction with the Lewis Acid Catalyst
AlCl3 is the lewis acid catalyst used here. It has a tendency to form [AlCl4]– complex by combining with a Cl– ion. Hence, AlCl3 gets attacked by the Cl atom in Formyl Chloride. The Formyl Cation has formed again, but this time it is free to react with aromatic compounds.
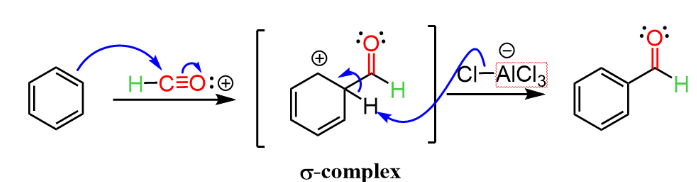
Step-3: Addition of the Formyl Cation to the Benzene Ring
The benzene is electron-rich due to the \(\pi\)-electrons. The benzene ring acts as a nucleophile and attacks the electrophilic carbon in Formyl Cation. This leads to the formation of a “\(\sigma\)-complex”. The [AlCl4]– formed in the previous step is converted back to AlCl3 by reaction with the \(\sigma\)-complex. The end product is the stable benzaldehyde.
Applications and Industry Use
The Gattermann Koch reaction is used in industry to synthesize aldehydes of Alkylbenzene. This reaction could not be used to react with non-alkylbenzenes.
There are two ways to carry out this reaction:
- At atmospheric pressure of CO with Cu2Cl2, ‘or’
- At high pressure of CO without Cu2Cl2.
FAQs
The Gattermann Koch reaction involves the formylation (addition of Aldehyde group) of an aromatic compound with the help of formyl chloride (produced by Carbon Monoxide and HCl) and Lewis Catalysts.
The reaction is performed either under pressure or using copper(I) chloride, which aids the formation of formyl chloride (HCOCl).
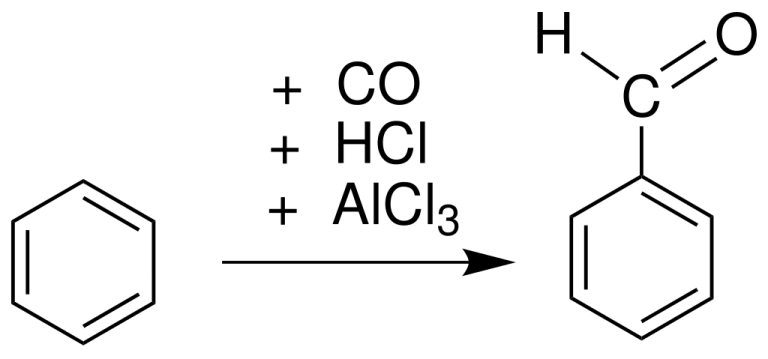
AlX3, FeX3 where X = Cl, F, I could be used as a lewis catalyst.
More Organic Reactions
