Cannizzaro Reaction is a chemical reaction that involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give primary alcohol and carboxylic acid.
Index
History
This reaction was first accomplished in 1853 by Stanislao Cannizzaro when he obtained benzyl alcohol and potassium benzoate from the treatment of benzaldehyde with potash.
More about Cannizzaro Reaction
This reaction is basically a reaction of aldehydes with caustic alkali in which one molecule of aldehyde is reduced to the corresponding alcohol and another molecule is oxidized to the salt of the corresponding acid.
The reaction is essentially a nucleophilic acyl substitution on an aldehyde where the leaving group attacks another aldehyde. The attack of hydroxide on carbonyl results in a tetrahedral intermediate.
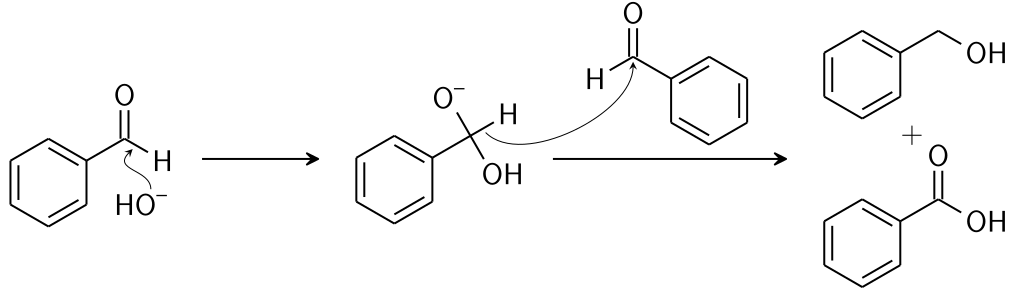
This reaction is a redox reaction involving the transfer of a hydride from one substrate molecule to the other: one aldehyde is oxidized to form the acid, the other is reduced to form the alcohol.
Cannizzaro Reaction Mechanism
Step 1: A nucleophile (hydroxide) is used to attack the carbonyl group of the given aldehyde. This causes a disproportionation reaction and also gives rise to an anion carrying 2 negative charges.
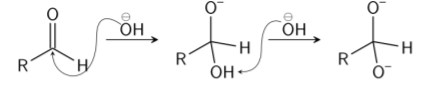
Step 2: The tetrahedral intermediate that results, then collapse, re-forming the carbonyl and transferring hydride to attack another carbonyl.
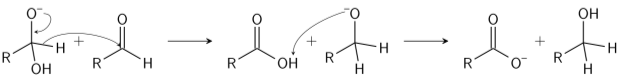
Step 3: In the final step, the acid and alkoxide ions that were formed, exchange a proton. The aldehyde first dorms a doubly charged anion, in the presence of a very high concentration of base, from which a hydride ion is transferred to the second molecule of aldehyde to form carboxylate and alkoxide ions. The alkoxide ion acquires a proton from the solvent, subsequently. Thus forming an acid and an alcohol
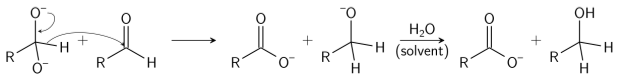
The reaction follows third-order kinetics.
Crossed Cannizzaro Reaction
At ideal conditions, the original reaction produces only 50% of the required alcohol and carboxylic acid. In order to achieve a better product, crossed Cannizzaro reaction is used. A sacrificial aldehyde is combined with a more valuable chemical and formaldehyde is used as a reductant, thus oxidizing it to sodium formate.
The alcohol is obtained from the reduction of the other aldehyde chemical. The yield of the valuable chemical is increased as 2 different aldehydes can be completely converted into the required product.
Applications of the Reaction
A crossed Cannizzaro reaction and aldol condensation are together used to prepare polyols in the industry. Different forms of polyols are used in polyesters for resins, synthetic lubricants, as a raw material in the varnish industry, etc.
FAQs
The reaction of aldehydes with caustic alkali in which one molecule of aldehyde is reduced to the corresponding alcohol and another molecule is oxidized to the salt of the corresponding acid.
Example of the Reaction:
2C6H5CHO + KOH → C6H5CH2OH + C6H5COOK
It is used to improve the yield of the desired product.
More Organic Reactions
