Surface Tension is a measure of a liquid’s tendency to minimize its surface area. Dimensions of surface tension can be derived from its definition.
It can be defined as the force acting per unit length on a line drawn tangential to the surface.
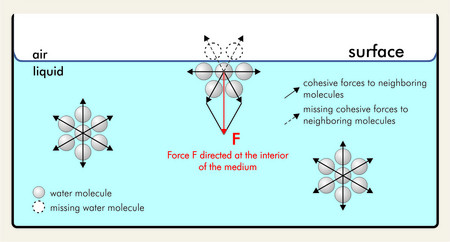
It is a common property of liquids and has many daily-life applications.
Index
Units of Surface Tension
As we can see, Surface Tension is defined as force per unit length. Thus, its units must be “Newtons per meter” in SI units. In CGS units, its units must be “dyne per cm”.
Dimensions of Surface Tension
We can derive the dimension of surface tension from its units.
We know the dimensions of force are \([\mathbf{M L T}^{-2}]\).
Similarly, the dimensions of length are \([\mathbf{M}^0 \mathbf{ L }^1 \mathbf{T}^0]\).
Thus, noting that Surface Tension = Force/Length, we get,
Dimensions of surface tension = \(\frac{[\mathbf{M L T}^{-2}]}{[\mathbf{M}^0 \mathbf{ L }^1 \mathbf{T}^0]} = [\mathbf{M} \mathbf{ L }^0 \mathbf{T}^{-2}]\)
Thus, dimension of surface tension are \([\mathbf{M} \mathbf{ L }^0 \mathbf{T}^{-2}]\).
FAQs
The dimensions of surface tension are \([\mathbf{M} \mathbf{ L }^0 \mathbf{T}^{-2}]\)
Surface tension is a property of a liquid. It measures how much a liquid tends to resist external forces. It is also a measure of how much the liquid tries to minimize its surface area.
Some daily-life examples of surface tension are:
1. Blowing bubbles takes work, due to surface tension of soap water.
2. A pin can float on water, supported by surface tension.
3. Water and other liquids can soak paper due to capillary action of fibres. This happens due to surface tension.
4. Bubbles and drops are round in shape because surface tension forces them to minimize surface area.
Surface tension is caused due to interaction of liquid molecules at the surface of liquid. The attraction they experience towards the interior is experienced as surface tension.
