Grignard reagent or Grignard compound is a class of chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl.
The French chemist Victor Grignard discovered Grignard Reagents. In 1900 after publishing his work, he received the Nobel Prize in Chemistry in 1912 for his work on organometallic compounds.
Grignard was working on the synthesis of organic compounds and fall upon the reaction between magnesium and alkyl halides, which led to the formation of a new class of compounds.
He later discovered that these compounds were highly reactive and could be used to create new carbon-carbon bonds. It led to the development of the Grignard reaction.
Index
Explanation
A Grignard Reagent is a type of organometallic compound that is formed by adding the halogenoalkane to small bits of magnesium in a flask containing ethoxyethane (commonly called diethyl ether or just “ether”) .
A reflux condenser, which is often used in organic chemistry to prevent the loss of reactants or solvents during a heated chemical reaction, is attached to the flask. The mixture is then heated over a water bath for a period of 20 to 30 minutes which leads to the formation of Grignard Reagent.
A Grignard reagent is generally represented by the general formula R-Mg-X, where R represents an alkyl or aryl group, X denotes a halogen atom like Cl, Br, or I, and Mg represents the magnesium atom.
A common Grignard reagent can be CH3CH2MgBr.
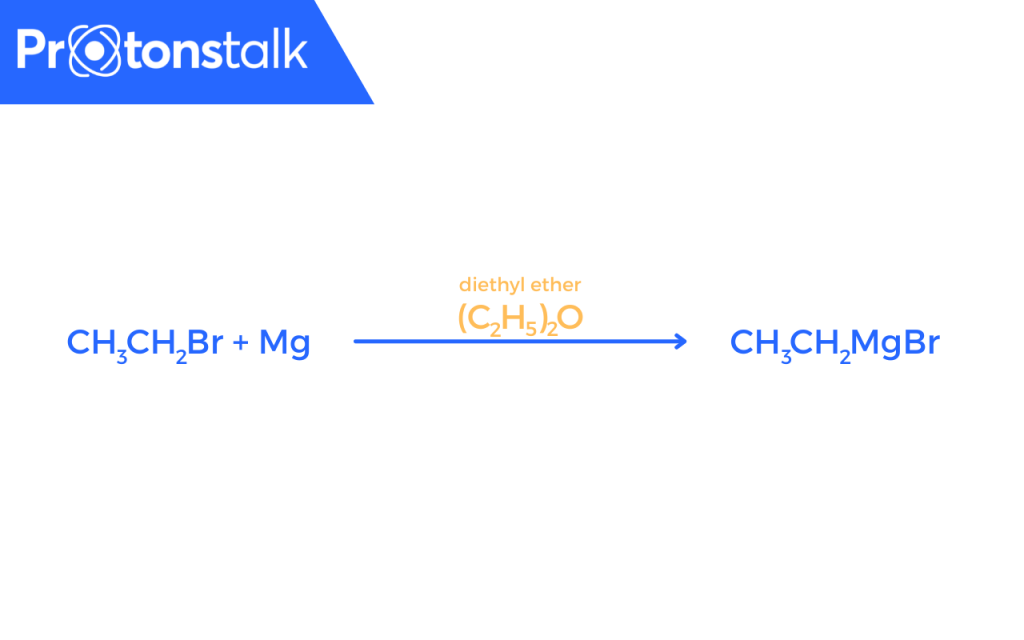
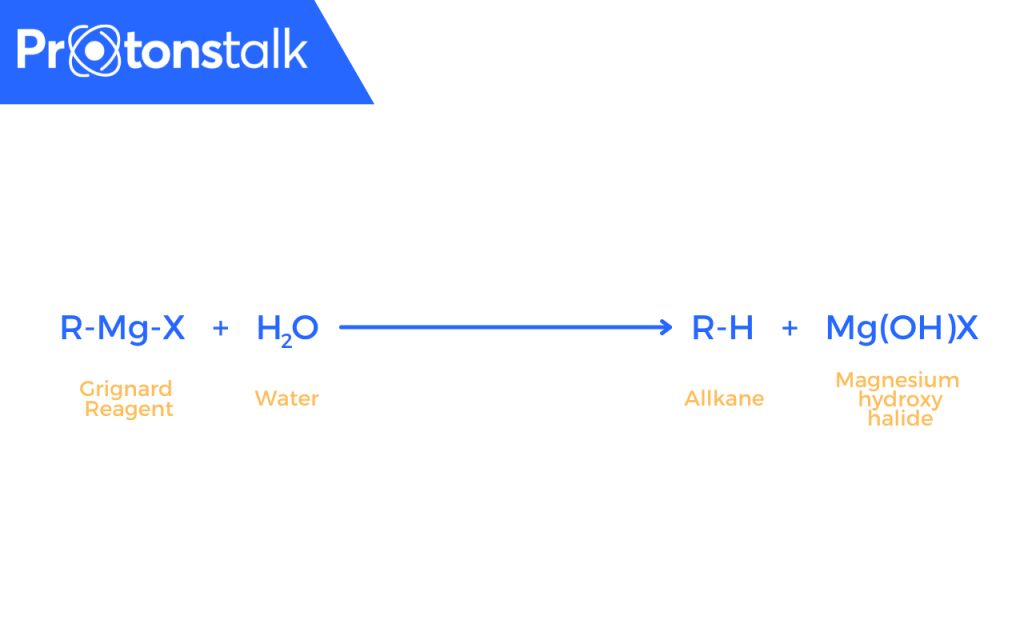
Due to reactivity of Grignard Reagents with water, it is necessary to ensure that everything must be perfectly dry.
As it reacts with water to produce alkane(as given below), that’s why it’s necessary that everything has to be very dry during the preparation of the Grignard reagent.
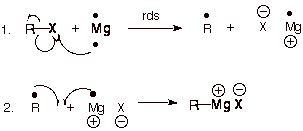
Formation Mechanism
There are mainly two steps:
- Reduction: The initial stage, which is the rate-determining step, comprises the transfer of a single electron from Mg, which has (two electrons in its valence shell), to the carbon-halogen bond. This results in the formation of a radical called Mg+1.
- Recombination: And in the second step, alkyl radical which formed in the first step undergoes a nucleophilic substitution reaction with the magnesium complex, resulting in the formation of the Grignard reagent.
Some Famous Reactions
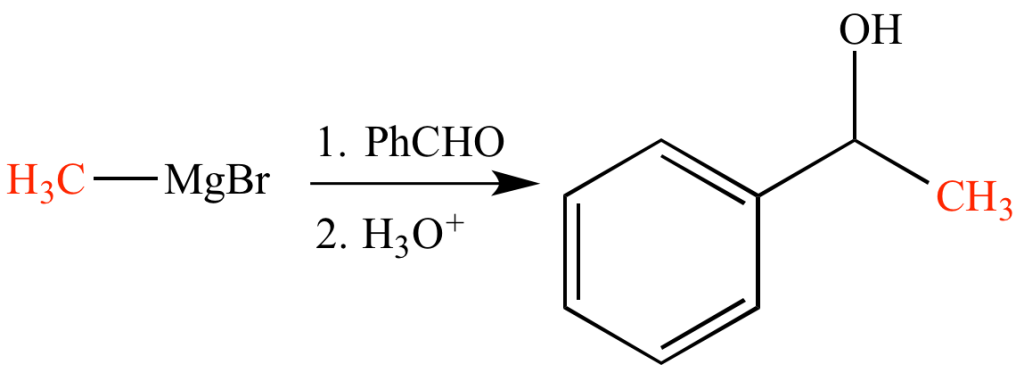
Reaction with Carbonyl Group
Grignard reagents (R–Mg–X) can react with various carbonyl compounds to form different products. One of the most common reactions involving Grignard reagents is the alkylation of aldehydes and ketones.
Reaction with Organic Halides
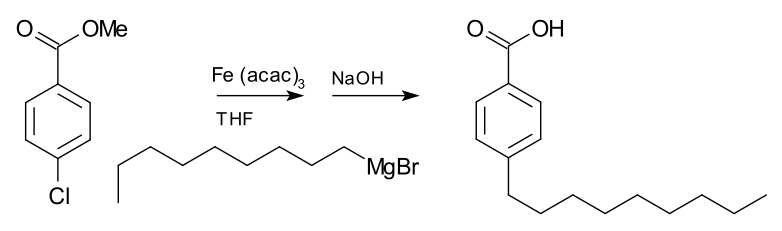
Although Grignard reagents exhibit high reactivity towards main group halides, they typically do not react with organic halides. However, in the presence of metal catalysts like nickel chloride and tetrahydrofuran (THF), coupling of aryl halides with aryl Grignard reagents takes place.
Following is one such reaction between methyl p-chlorobenzoate and nonyl magnesium bromide which yields the compound 4-nonyl benzoic acid in the presence of the catalyst – Tris(acetylaceto) iron(III).
Industrial Use
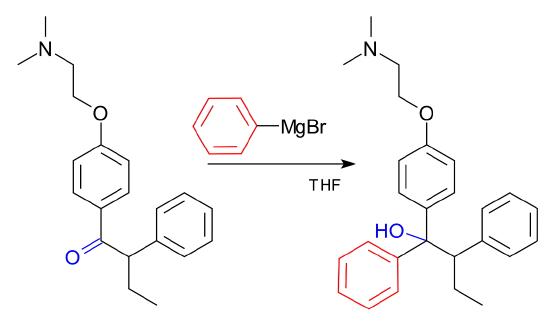
the Grignard reagent plays a crucial role in the non-stereoselective (set of reactants combine to form a racemic mixture) production process of Tamoxifen. This medication is used for the prevention and treatment of breast cancer.
Reaction with CO2
A famous reaction of the reagent is where it reacts with dry ice (solid CO2) with aqueous acid work-up to give carboxylic acids.
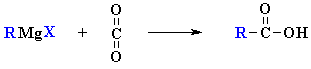
Applications
Some of the important applications:
- Grignard reagent plays an important role in organic chemistry due to its ability to participate in reactions that lead to the formation of new carbon-carbon bonds.
- It is used to determine the number of halogen atoms present in the halogen compound.
- It is also used to manufacture chemo-catalysts, amides, acetals, amino compounds, organosulfur compounds, ethers, ketones, aldehydes, etc. These are used for the production of several compounds having important applications in pharmaceuticals, perfumes, and other excellent or specialty chemicals.
FAQ’S
It is a chemical compound with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl.
Yes, we can use grignard reagent to prepare primary, secondary and tertiary alcohols.
Methyl alcohol
As C-X bond energy is maximum in CH3F hence, fluoride is less reactive to form the grignard reagent with Mg.
Due to the formation of respective alkane, it is not used as a solvent for grignard reagents.
Diethyl ether or Tetrahydrofuran (THF)
Related Topics
