Friedel Crafts Acylation, as the name suggests, is an organic reaction that is used in the addition of acyl substituents to aromatic compounds. This is a coupling reaction that uses electrophilic aromatic substitution in order to substitute the hydrogen atoms present on the aromatic compounds with acyl groups.
Index
History
The Friedel crafts acylation reaction was developed in 1877, by Charls Friedel a French chemist and James Crafts, an American chemist.
More About Friedel Crafts Acylation
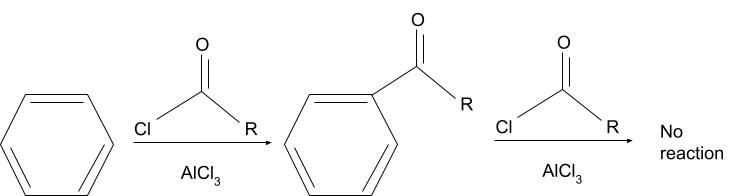
The reaction in which, hydrogen on an aromatic compound is substituted with an acyl group in the presence of an acyl donating group and a lewis acid is the Friedel crafts acylation reaction. After the reaction is complete, the aromatic compound is usually converted into a ketone.
The commonly used reagents used in this reaction are AlCl3 for the lewis acid and Acyl Halides for the acyl group. But acid anhydrides are also used in the place of acyl halides.
In the Friedel crafts acylation reaction, the major process involved is electrophilic aromatic substitution. The electrophile which needs to be present for the reaction to occur is formed in the first step of the reaction, where a complex is formed between the lewis acid and the acyl donor (either an acyl halide or an acid anhydride).
During the reaction, the aromatic compound loses its aromaticity temporarily when the acyl group is added to it.
Friedel Crafts Acylation Mechanism
In order to depict the reaction occurring in each step of the Friedel crafts acylation reaction, let us use the example of friedel crafts acylation of benzene with AlCl3 being the lewis acid catalyst.
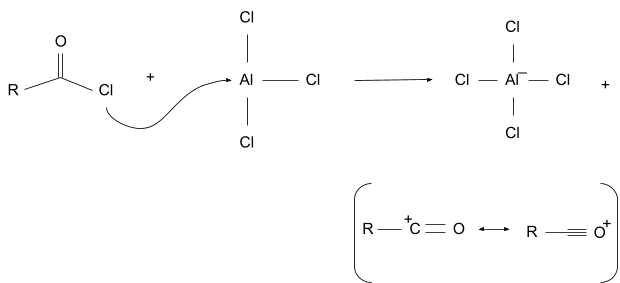
1. Electrophile Formation
In this the acyl donor first forms a complex with the lewis acid, then the acyl part of the complex breaks free and forms an acyl ion. The acyl ion is stable due to resonance.
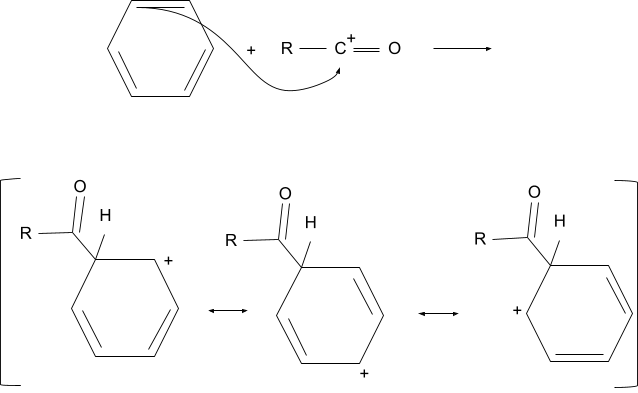
2. Addition of the Acyl Group
After the formation of the acyl ion, it attacks the aromatic compound. After attacking the compound loses its aromaticity and a positive charge is formed which is distributed in the compound due to its conjugation with double bonds.
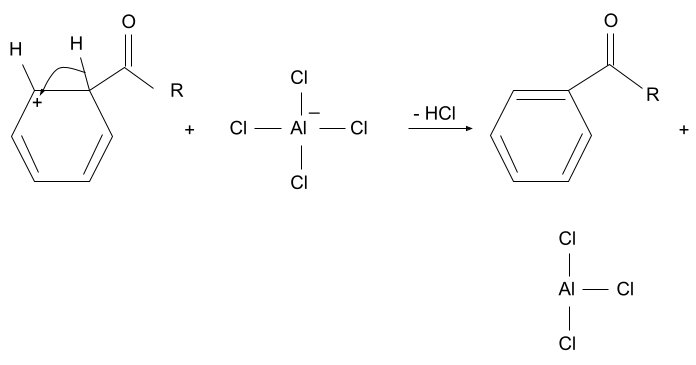
3. Deprotonation
The hydrogen present along with the acyl group is removed from that position by the complex which formed in the first step when the acyl ion was formed. When the hydrogen is removed, the aromaticity of the compound is restored and the acyl group is substituted.
Limitations of Friedel Crafts Acylation
- Product Limitation
Only ketones can be produced using this reaction, as formyl chloride, which must be used if one wants to produce aldehydes, decomposes under the reaction conditions of friedel crafts acylation.
- Catalyst
The finished products which are acylated aromatic compounds, form a stable complex with the lewis acid catalyst and at the end of the reaction aluminium salts are formed from the compound. Due to this, a stoichiometric amount of lewis acid must be used in the reaction as unlike in friedel crafts acylation it is not regenerated at the end of the reaction.
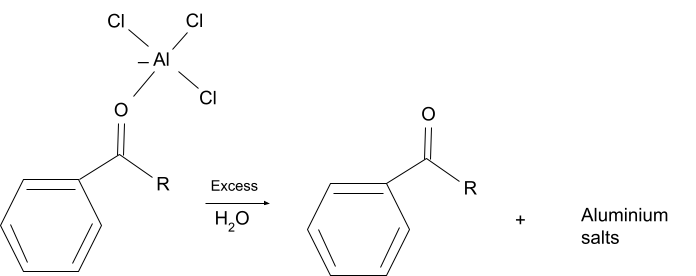
- Functional Group Restriction
In a friedel crafts acylation reaction aryl amines cannot be used, as they form very stable/unreactive complexes with the lewis acid used as catalyst.
- Reactivity
If the aromatic compound used in the reaction is less reactive than a mono-halobenzene, then it cannot participate in it.
- Electron Density on the Aromatic Compound
The position and rate of substitution of the acyl group depends on the electron density of the aromatic compound which is influenced by the substituents present on it. The electron density in a position of the aromatic ring also influences the substitution position.
- Other Factors due to Substituents on the Aromatic Compounds
The substituents besides activating or deactivating an aromatic compound, influence the reaction in various ways:- Steric Factors
When the substituent groups on an aromatic compound are bulky(occupy more space), the substitution usually takes place away from them. - Acidic Hydrogens
The acidic hydrogens in the substituents present on an aromatic compound also react with the negatively charged lewis acid. - Lone Paris
If there are lone pairs of electrons present on the atoms of a substituent then there is a chance of the electrophile attacking them instead of the aromatic compound. - Stability Factors
The position of substitution can also vary depending on the stability of the substituents present on the aromatic compound. Eg. If there are two substituents on an aromatic compound and they are stable due to chelation, then the position of substitution changes so that it does not interfere with their chelation.
The acyl group is substituted where it is relatively more stable with substituents around it. Eg. if in one position the acyl group forms a chelation with the nearby substituent and in another position it does not, then it is substituted in the chelation forming position.
- Steric Factors
Applications of Friedel Crafts Acylation
- One of the most important uses of this reaction is that it is used in the production of dyes, like xanthene dyes and thymolphthalein (indicator).
- It is also used in Haworth reactions.
- It is used in synthesising aromatic compounds which have ketone and aldehyde functional groups. By combining this reaction with some other organic reactions even functional groups like carboxylic acid can be added to an aromatic compound.
Example Problems
Question 1. What will be the product for following reaction?
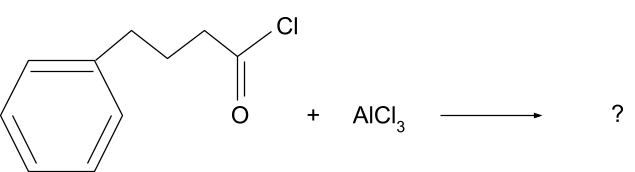
Solution.
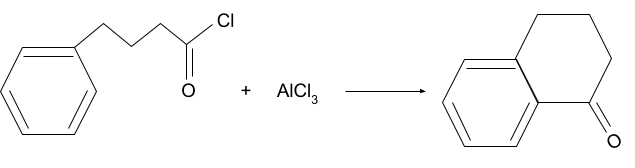
Question 2. What will be the product for following reaction?
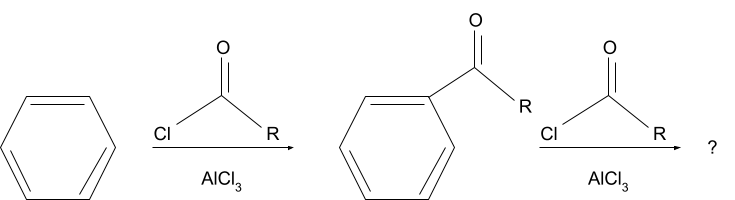
Solution.
FAQs
Friedel crafts acylation is an organic coupling reaction in which an acyl group is added to an aromatic compound by using a lewis acid as a catalyst and an acyl halide or an acid anhydride as the acyl group donor.
After the completion of the reaction the lewis acid which is present in the reaction solution reacts with the substituted aromatic compound and forms a complex which then reacts with excess water to form salts of aluminium. So, due to the formation of aluminium salts the catalyst is not regenerated after the completion of the reaction, due to which enough catalyst must be present at the beginning of the reaction.
Friedel crafts acylation reaction which takes place within the aromatic molecule with one of its substituents as the acyl donor.
Some of the factors which influence the substitution position of the acyl group are:
1. Steric factors
2. Ring activation and deactivation
3. Stability factors
4. Reactivity
More on Organic Reactions