Friedel-Crafts Alkylation is, as the name suggests, the alkylation of an aromatic compound. This is a coupling reaction, in which, the hydrogen in an aromatic compound is substituted with an alkyl group using electrophilic aromatic substitution.
Index
History
Friedel-crafts alkylation was developed in 1877, by Charls Friedel and James Crafts to attach substituents to an aromatic compound.
Charles Friedel was a French chemist and mineralogist who was awarded the Davy Medal. James Mason Crafts was an American chemist who was recognized by the french government which made him a chevalier of the Legion of Honor apart from this, he was also made a member of the British Association for the Advancement of Science also Harvard university granted him the Honorary Degree of LL.D.
More About Friedel-Crafts Alkylation
Friedel-crafts alkylation at its core is a substitution reaction, it involves the substitution of a hydrogen atom in an aromatic compound with an alkyl group in the presence of a strong lewis acid which acts as a catalyst.
In Friedel-crafts alkylation, the alkyl group which is to be substituted is always an alkyl halide, and the strong lewis acid which is used as the catalyst is mostly AlCl3 , but FeCl3 is also used almost just as frequently.
An electrophilic aromatic substitution reaction occurs during a friedel-crafts alkylation reaction. In that reaction, an electrophile is formed from a reaction between alkyl halide and lewis acid, where the lewis acid removes the halide in the alkyl halide thereby creating a carbocation which acts as an electrophile.
Friedel-Crafts Alkylation Reaction Mechanism
The Friedel-crafts alkylation reaction can be divided into three well defined steps or stages through which the reaction occurs. For the representation of reactions at each step in the mechanism we take the example of the friedel-crafts alkylation of benzene.
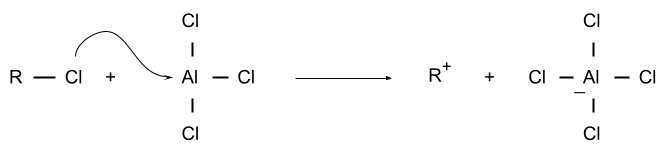
1. Formation of Electrophile or Carbocation
This is the step in which an electrophile is formed by the reaction between alkyl halide and lewis acid.
The electrophile formed is a carbocation of an alkane.
The reaction involved is,
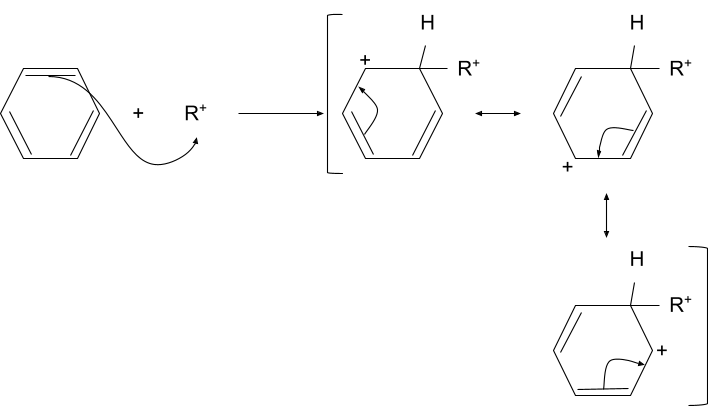
2. Electrophilic Addition
In this step the electrophile formed in the previous step attacks the aromatic compound and so it temporarily loses its aromaticity. The intermediate formed in this step is resonance stabilized by the double bonds present in the aromatic compound.
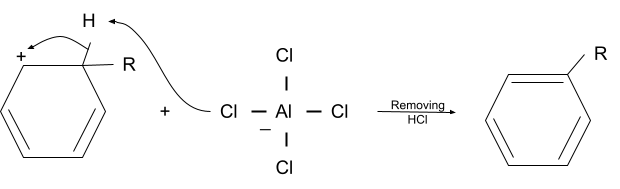
3. Deprotonation
The last step of the friedel-crafts alkylation reaction mechanism is deprotonation. The hydrogen initially present where the alkyl group is added is removed by the negatively charged lewis acid. So, the substitution of the alkyl group in an aromatic compound is complete.
Limitations of Friedel-Crafts Alkylation
- Carbocation Rearrangement
When alkyls of more than 2 carbons are added to the aromatic compound there is a possibility of occurrence of a carbocation rearrangement and can lead to a different product than expected. The rearrangement can also occur in the aromatic compound.
- Electron Density on the Aromatic Compound
When there are substituents like nitro or ammonia which deactivate or activate, respectively, the aromatic compound, the position of electrophilic aromatic substitution taking place in the reaction changes according to the electron density in the various positions of the aromatic compound.
The reactivity of the aromatic compound also changes based on the overall electron density on it.
- Waste Products
Due to the alkyls formed during the reaction sequence being highly reactive, many side reactions occur which produce many waste products. The side reactions are usually the carbocation attacking the finished products which are slightly activated due to the presence of an alkyl group.
- Reactivity
The aromatic compounds which are less reactive than halobenzenes do not undergo this reaction.
- Other Factors due to Substituents on the Aromatic Compounds
The substituents besides activating or deactivating an aromatic compound, influence the reaction in various ways:- Steric Factors
When the substituent groups on an aromatic compound are bulky(occupy more space), the substitution usually takes place away from them. - Acidic Hydrogens
The acidic hydrogens in the substituents present on an aromatic compound also react with the negatively charged lewis acid. - Lone Paris
If there are lone pairs of electrons present on the atoms of a substituent then there is a chance of the electrophile attacking them instead of the aromatic compound. - Stability Factors
The position of substitution can also vary depending on the stability of the substituents present on the aromatic compound. Eg. If there are two substituents on an aromatic compound and they are stable due to chelation, then the position of substitution changes so that it does not interfere with their chelation.
- Steric Factors
Applications of Friedel-Crafts Alkylation
- It is used in making many different kinds of aromatic compounds with different substituents.
- This reaction is related to several classic named reactions like Clemmensen Reduction.
- It is used in the synthesis of dyes called Triarylmethane (CH(C6H5)3).
- This reaction is used in testing for aromatic compounds.
Example Problems
Question 1. What will be the product for following reaction?
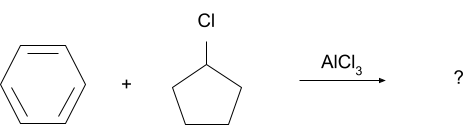
Solution.
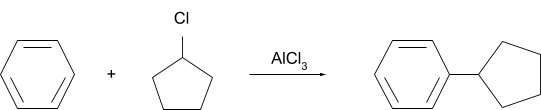
Question 2. What will be the product for following reaction?
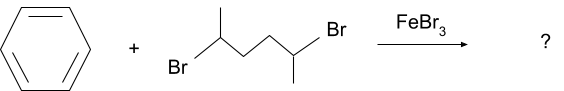
Solution.
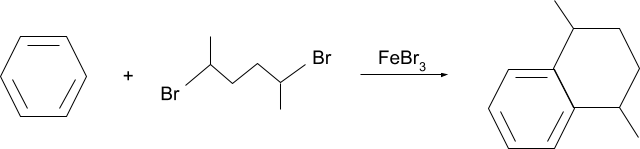
FAQs
When the friedel-crafts alkylation reaction occurs within the aromatic molecule between the main molecule and one of its substituents, it is called an intramolecular friedel-crafts reaction.
Example.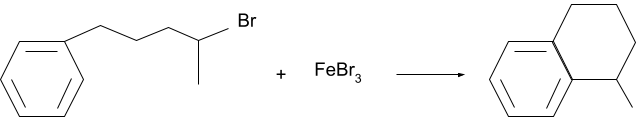
The reaction in which alkyl groups are added to aromatic compounds using the method of electrophilic aromatic substitution and lewis acid as a catalyst is called friedel-crafts alkylation.
Some of the common catalysts used in a friedel-crafts alkylation reaction are:
1. AlCl3
2. FeCl3
3. H2SO4 (depending on the group contained by the alkane to be substituted)
In the friedel-crafts alkylation reaction as the attacker is an electrophile it attacks the position where the electron density is high.
In an activated ring the electron density is more making the aromatic compound more reactive, whereas in a deactivated ring, the electron density is low, thereby making it less reactive. So the reaction rate is high in an activated ring and low in a deactivated ring.
More on Organic Reactions