Thymolphthalein is an acid-base indicator. It changes colour based on the substance it is added to and indicates whether it is an acid or base.
Index
Usage as an Indicator
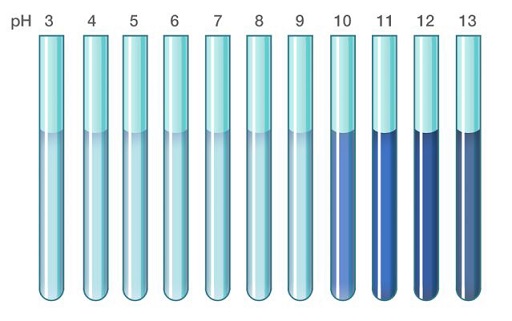
When used as an indicator, it behaves as follows:
- Below pH 9.3 – 10.5, it is colourless.
- Above pH 9.3-10.5, it is blue in colour.
Thus, it undergoes a transition between pH 9.3-10.5.
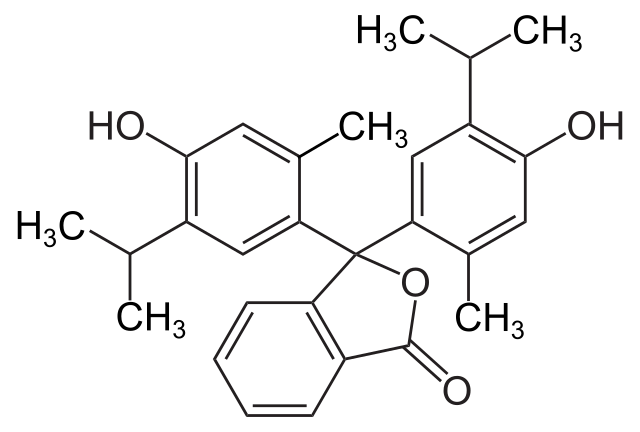
Structure of Thymolphthalein
We have the structure of chemicals as follows:
Other Uses of Thymolphthalein
Other than being used as an indicator, it can be used in:
- Disappearing ink
- The Kastle-Mayer test for blood
- Making of coloured bubbles
- Laxatives
Obtaining Thymolphthalein
The chemical can be obtained in the following ways:
Previously Synthesized
It can be obtained in powder form. Then it is dissolved in ethanol solution which is then diluted with water.
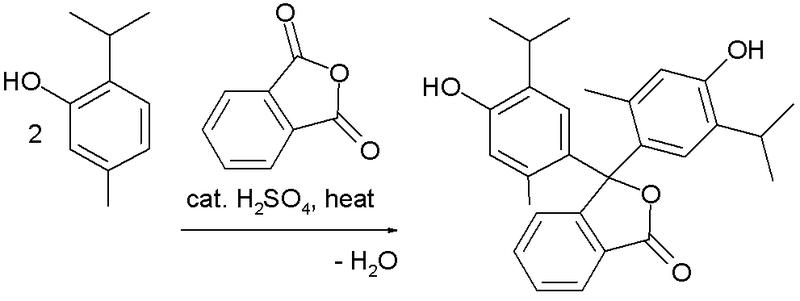
Own Synthesis
It can be synthesized by a reaction between thymol and phthalic anhydride.
FAQs
It has the following uses:
– Used as an indicator for acids and bases
– Also used as a laxative
– It is used in making coloured bubbles
– Used as disappearing ink
– Also used for a test for blood.
It turns colourless in acids, and blue coloured in bases.
No, it is different from phenolphthalein. However, both are acid-base indicators and are from related chemical families.
Know more on Phenolphthalein
The acid-base transition pH of the indicator is 9.3-10.5 pH.
Related: Methyl Orange
