Zinc acetate is a salt with the chemical formula Zn(CH3CO2)2 (or) C4H6O4Zn. It generally occurs as a dihydrate. Hence the formula is written as Zn(CH3CO2)2.2H2O.
Both the anhydrous and hydrate form of the compound are colorless.
Index
Structure and Formula
Chemical formula of zinc acetate (anhydrous) is Zn(CH3CO2)2 and its structure is as follow,
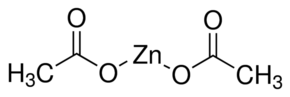
The zinc is coordinated to four oxygen atoms to give a tetrahedral shape. These tetrahedral polyhedral are interconnected by acetate ligands to give a range of polymeric structures.
The chemical formula for the hydrate form is Zn(CH3CO2)2.2H2O and has a structure as follow,
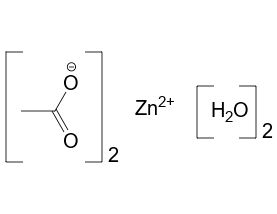
The zinc here is octahedral. And both the acetate groups are bidentate.
Properties
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
So, here are the physical properties of the compound Zn(CH3CO2)2
| Molecular weight (anhydrous) | 183.50 g/mol |
| Molecular weight (dihydrous) | 219.50 g/mol |
| Density (dihydrate) | 1.74 g/cm3 |
| Melting point | 273°C |
| Boiling point | decomposes |
| Appearence | Colorless crystalline solid |
| Odour | Faint acetic |
| Water solubility | 43g/100ml |
| Molecular shape | Tetrahedral |
Chemical Properties
Following are the chemical properties of the compound Zn(CH3CO2)2
- Flammability = Non flammable or Combustible
- Toxicity = Mildly toxic (humans)
- pH value = 5 – 6
- Standard enthalpy of formation (dihydrous) = (1669.35 ± 1.30) kJ · mol
Zinc ion(II) reacts with aq. Ammonia to precipitate and give a white gelatinous Zn(OH)2.
\(Zn^{+2}(aq) + 2NH_3(aq) + 2H_2O(l) \rightleftharpoons Zn(OH)_2(s) + 2NH_4^{+}(aq)\)

Preparation of Zinc Acetate
It is prepared using either Zn(Zinc) or ZnO(Zinc oxide)
\(2CH_3COOH + Zn \rightarrow Zn(CH_3COO)_2 + H_2\)
\(2CH_3COOH + ZnO \rightarrow Zn(CH_3COO)_2 + H_2O\)
To prepare anhydrous Zinc Acetate, we firstly dry the dihydrate form by refluxing with toluene and then separate water using Dean-Stark Apparatus.
Applications of Zinc Acetate
- The compound has been used in lozenges for treating the common cold.
- It can also be used to treat zinc deficiencies it also plays a part in the treatment of Wilson’s disease.
- It is also used in everyday laboratory uses.
FAQs
Zinc Acetate Formula
Anhydrous – \(Zn(CH_3COO)_2\)
Dihydrate – \(Zn(CH_3COO)_2 . 2H_2O\)
Zinc acetate (Zn(CH3CO2)2) is an ionic compound.
It is used to treat and prevent zinc deficiency as zinc is essential for the growth and health of body tissues. It has been used in lozenges for treating the common cold. It is also used in a lot of everyday laboratory uses
More on Inorganic Compounds
