Dinitrogen Trioxide (N2O3) is a deep-blue solid compound. It is formed upon mixing equal parts of nitrogen dioxide and nitric oxide and cooling the mixture below – 21℃.
Index
Structure and Formula
The chemical formula for Dinitrogen Trioxide N2O3. Its structure is as follows,
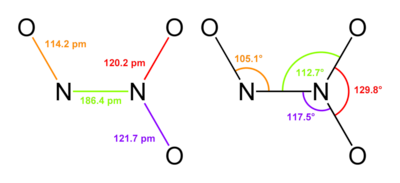
The N-N bond has length 186pm. The molecule is planar and exhibits Cs symmetry.
Properties of Dinitrogen Trioxide
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
The compound has the following physical properties,
| Appearance | Deep blue |
| State of matter | Gaseous state |
| Solubility | Soluble in water, ether |
| Melting point | -100.7℃ |
| Boiling point | 3.5℃ |
Chemical Properties
The chemical compound has the following chemical properties,
- Chemical formula – N2O3
- Molar mass – 96.01 g/mol
- Density – 1.783 g/cm3 (gas), 1.4g/cm3 (liquid)
Production of Dinitrogen Trioxide
The chemical compound can be produced by mixing equal parts nitric oxide and nitrogen dioxide and then cooling this mixture below – 21℃.
NO + NO2 → N2O3
Applications of Dinitrogen Trioxide
- N2O3 is used in special-purpose fuels.
- It is a powerful oxidizer, and if combined with other chemical compounds, it can be used as an oxidizing agent.
- It finds applications in the chemical industry, such as in making dyes, nylon, etc.
Hazards
- The chemical compound, N2O3 is highly toxic.
- It may explode if heated.
- It causes fatal effects if it comes in contact with skin, or inhaled and can also cause severe eye damage and skin burns.
FAQs
The chemical formula of the coumpound is N2O3.
It is a covalent compound.
It is acidic in nature.
More on Inorganic Compounds
