Barium Carbonate (BaCO3) is a white crystalline inorganic compound. This is an alkali metal carbonate, which is a white powder-like salt and is poorly soluble in water. Barium carbonate’s formula is BaCO3 and is also called Barium Monocarbonate.
Index
History
Barium carbonate exists in nature as the mineral witherite. This mineral was first discovered by William Withering in the year 1784 from barytes. The mineral witherite was named after William Withering who discovered it.
Withering discovered this chemical from the experiments he conducted on a heavy metal ore found in Cumberland(North west England) called terra ponderosa.
One of the earliest samples of witherite may be in the Matthew Boulton mineral collection of Birmingham Museum and Art Gallery. The chemical witherite later came to be known as barium carbonate.

Structure and Chemical Formula
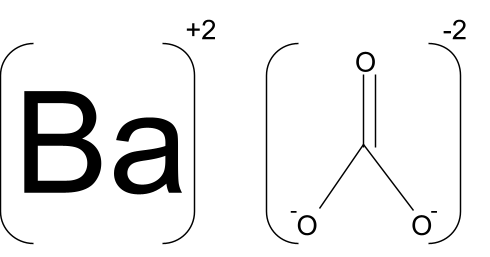
Barium carbonate is an ionic compound with +2 charge on the cation and -2 charge on the anion.
Properties of Barium Carbonate
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
| Appearance | White Powder (Crystalline Solid) |
| Density | 4.286 g/cm3 |
| Melting Point | 811°C (Polymorphic Transformation) |
| Boiling Point | 1450°C, Starts decomposing from 1360°C |
| Solubility | Insoluble in methanol; Partially insoluble in water; Decomposes in acids |
| Heat Capacity | 85.35 J/mol.K |
| Odor | Odorless |
| Refractive Index | 1.676 |
Chemical Properties
- When soluble calcium salts react with BaCO3 they form a barium salt that remains in the solution itself.
An example:
BaCO3 + CaSO4 -> BaSO4 + CaCO3.
- It reacts with some acids to form soluble salts. An example of this is its reaction with hydrochloric acid.
BaCO3 + 2HCl -> BaCl2 + CO2 + H2O.
- The pyrolysis of BaCO3 gives barium oxide.
Production
BaCO3‘s most important chemical form is the mineral witherite which is found naturally, when seen from a commercial point of view, but it is also prepared from barytes. Witherite is generally found in the veins of lead ores.
From Barium Sulfide
- Barium carbonate is prepared from barium sulfide for its commercial purposes. Barium sulfide is treated with sodium carbonate at 60°C to 70°C, this is called the soda ash method.
The more commonly used method is the carbonation method, where barium sulfide is treated with carbon dioxide at 40°C to 90°C instead of sodium carbonate.
BaS + H2O + CO2 -> BaCO3 + H2S
- It is also prepared from barium sulfide also by a metathesis reaction with ammonium carbonate. This method is called the metathesis method.
BaS + (NH4)2CO3 -> BaCO3 + (NH4)2S
From the Mineral Witherite
Barium carbonate is also prepared from refining the mineral witherite. This is done by first reacting witherite with an ammonium salt which produces a soluble barium salt, then again reacting the formed ammonium carbonate with the barium salt to form refined BaCO3.
BaCl2 + NH4HCO3 + NH4OH -> BaCO3 + 2NH4Cl + H2O
Applications of Barium Carbonate
- Used to remove the sulfate impurities present in the feedstock of chlor-alkali process.
- It is also commonly used in the preparation of barium containing compounds like ferrites.
- Used as an ingredient in glazes in the ceramic industry.
- It also acts as a flux, and as a matting and crystallizing agent also.
- Produces unique colours by combining with some colouring oxides.
- Also used to precipitate soluble salts that cause efflorescence in brick, tile, earthenware and pottery industries.
- Used as a raw material for the production of barium oxide and peroxide.
- It is also used in other industries like photography, oil drilling, barium magnetic materials, paint, chemical industry etc.
- Also used in the manufacture of electrical goods like capacitors, PTC thermistors, electronic ceramics and many others.
- It is one of the important raw materials for the production of fibre optical glass.
Hazards
- It is a highly toxic chemical and it needs to be handled very carefully. It must always be kept at a low concentration at about 20%.
- The use of this inorganic compound in making various colours of glazes in the ceramic industry is still controversial as some think that it might leach from glazes into food. So to provide a safe means of use barium oxide is used in fritted form.
FAQs
BaCO3 is the chemical formula for barium carbonate.
Witherite is one of the most commercially valuable compound of barium.
BaCO3’s compound name is barium carbonate
More on Inorganic Compounds
