Chlorine Trifluoride (ClF3) is an interhalogen compound. The chemical formula is ClF3. It is a powerful oxidizer and it ignites highly combustible materials spontaneously.
It was first reported by Ruff and Krug in 1930, who prepared it by fluorination of chlorine.
Index
Structure & Formula
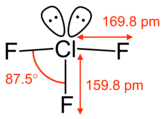
The structure of chlorine trifluoride is shown above. The molecular geometry is approximately T-shaped, with a short bond and two long bonds.
The chemical formula is ClF3. Its IUPAC name is trifluoro-λ3-chlorane. It is also known as Chlorotrifluoride.
Properties of Chlorine Trifluoride
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
Physical properties of this compound are:
- It has a pungent odor.
- Exists in the gaseous state and is colorless.
- It is pale green-yellow in color when in a liquid state.
- This coumpound has an atom count of 4 and a complexity of 8.
- It is soluble in water.
- It is colorless, poisonous, and corrosive.
Chemical Properties
The chemical properties for this compound are as follows:
- Its chemical formula is ClF3.
- It has a density of 3.779 g/L.
- Its melting point is -76.34℃ and its boiling point is 11.75℃.
- It has a heat capacity of 63.9 J K-1 mol-1.
- Gibbs free energy is -123.0 kJ mol-1.
Production of Chlorine Trifluoride
The compound can be produced using the simple reaction,
3F2 + Cl2 → 2ClF3
Applications of Chlorine Trifluoride
- It finds an application as a fluorinating agent.
- It is used as an igniter and propellant in rockets.
- It can also be used in nuclear fuel processing to convert uranium into gaseous hexafluoride uranium.
- ClF3 can also be used to clean chemical vapor deposition chambers in the semiconductor industry.
Hazards
Chlorine trifluoride is a very strong oxidizing and fluorinating agent. It is extremely reactive with most organic and inorganic materials. It has the capability of initiating the combustion of many otherwise non-flammable materials without any ignition source.
This coumpound has the ability to corrode non-corrodible materials such as iridium, platinum, and gold. It has been reported to ignite sand, asbestos, and other highly flame-retardant materials.
It can also ignite the ashes of materials that have already been burned in oxygen.
Exposure to huge amounts of ClF3 as a liquid or as gas may ignite living tissue.
FAQs
Chlorine trifluoride has been banned under the Chemical weapons Convention, after World War II.
It is a highly oxidizing agent, which has the ability to turn water into oxygen upon contact. It was produced by Nazis during World War II but was never used. This renders it a highly dangerous compound.
The only way to store it safely is to put it inside sealed containers made from steel, iron, nickel, or copper, after being treated with fluorine gas.
More on Inorganic Compounds
