Barium bromide (BaBr2) is an important chemical compound due to its use in the synthesis of various chemicals. It is toxic when dissolved in water.
This is a white crystalline compound and is deliquescent in nature. It acts as a simple salt when dissolved in water and reacts with various reagents accordingly.
Index
Structure and Formula
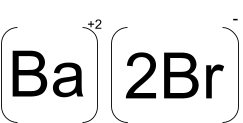
The chemical formula of barium bromide is BaBr2. It is also called barium(+2) dibromide and is the anhydrous form of the compound. There is also a hydrous form of the compound named barium bromide dihydrate, BaBr2.2H2O which actually crystallises and can be heated at 120°C to produce anhydrous form of the compound.
The crystals of this compound are white orthorhombic and deliquescent with the lead chloride motif. The nature of chemical bonds in this compound is clearly ionic due to its behaviour as a simple salt when dissolved in water.
Properties
Now, let’s move on to the discussion of properties of this compound which implies physical properties and chemical properties.
Physical Properties
| Barium Bromide Molar mass | 297.14 g/mol |
| Appearance | White crystalline solid |
| Density | 4.78 g/cm3(anhydrous); 3.58 g/cm3(dihydrate) |
| Melting point | 857°C |
| Boiling point | 1835°C |
| Barium Bromide Solubility | 92.2 g/100 ml (0°C) (water) |
Chemical Properties
It reacts with chemicals like oxalic acid, hydrofluoric acid, sulfate salts and phosphoric acid and gives their respective precipitates barium oxalate, barium fluoride, barium sulfate and barium phosphate.
For example,
BaBr2(aq) + SO4+2 (aq) -> BaSO4 (s) + 2Br– (aq)
Production
This compound BaBr2 is prepared by the reaction of barium sulfide or barium carbonate with hydrobromic acid.
BaS + 2HBr -> BaBr2 + H2S
BaCO3 + 2HBr -> BaBr2 + CO2 + H2On
BaBr2 crystals are formed from the concentrated solution of barium bromide dihydrate.
Applications of Barium Bromide
- The compound is used in the production of chemicals, used in photography.
- It is also used in the production of many other bromides.
- It was once used in the purification of radium through the method of fractional distillation.
- This compound is also used in the production of many phosphors.
Hazards
When dissolved in water it becomes toxic. Swallowing barium bromide can cause severe poisoning like paralysis. It can cause bromism that affects the central nervous system. May cause eye and skin irritation when exposed to it. Inhalation of high concentration can cause suffocation.
Wash eyes or skin continuously and thoroughly for at least 15 min. When inhaled, immediately take out to open air. Medical aid is must even after any of these measures.
FAQs
The chemical formula for barium bromide is BaBr2.
This compound when ingested causes nausea, vomiting and diarrhoea. It can also cause kidney, liver and spleen damage.
It never occurs in its pure form in nature because of its high reactivity with air. It even combines with chemicals like carbon, sulfur or oxygen to produce various barium compounds.
More on Inorganic Compounds
