Sodium sulfate (Na2SO4) is a highly water soluble white powder like chemical substance. There are also many other forms of sodium sulfate which vary in their water of crystallization.
The chemical formula of sodium sulfate is Na2SO4. This chemical can either be naturally found in the form of crystalline minerals and also in dissolved form in salt lakes, or it can be produced artificially as the by-product of reactions. Both of these have their own uses in commercial goods.
Index
History
Apothecary Johann Rudolf Glauber was a German/Dutch chemist and he was the one who discovered sodium sulfate in 1625 in the spring waters of Austria. He discovered decahydrate of this chemical which is now called Glauber’s salt or mirabilis, its chemical formula is Na2SO4.10H2O.
He named it sal mirabilis (miraculous salt) as it has medicinal values. Until the 1900s when more wide uses of this chemical were introduced it was used as a laxative.
It is also used in the production of soda ash (Na2CO3). Its use in the preparation of soda ash started in the 1700s, Glauber’s salt is used in the production process and reacted with potash to form soda ash. The increasing demand for soda ash led to the rise in the demand of this chemical as well, so, in the 1800s the Leblanc process was adapted in the large scale production of soda ash.

More about Sodium Sulfate
Sodium sulfate is a very widely used chemical, and its average production rate is 5.5 to 6 million tons per year, which makes it apparent how much this chemical is used. Around 2/3rds of this quantity is produced from mirabilis. The uses and names of this chemical change with the water of crystallization. There are primarily 3 forms:
- Anhydrous – Na2SO4, this is also called thenardite which is a mineral rarely found in nature.
- Heptahydrate – Na2SO4.7H2O, this is also a very rare form to occur naturally.
- Decahydrate – Na2SO4.10H2O, this is commonly known as Glauber’s salt or mirabilis, but is called mirabilite when found in the mineral state in nature.
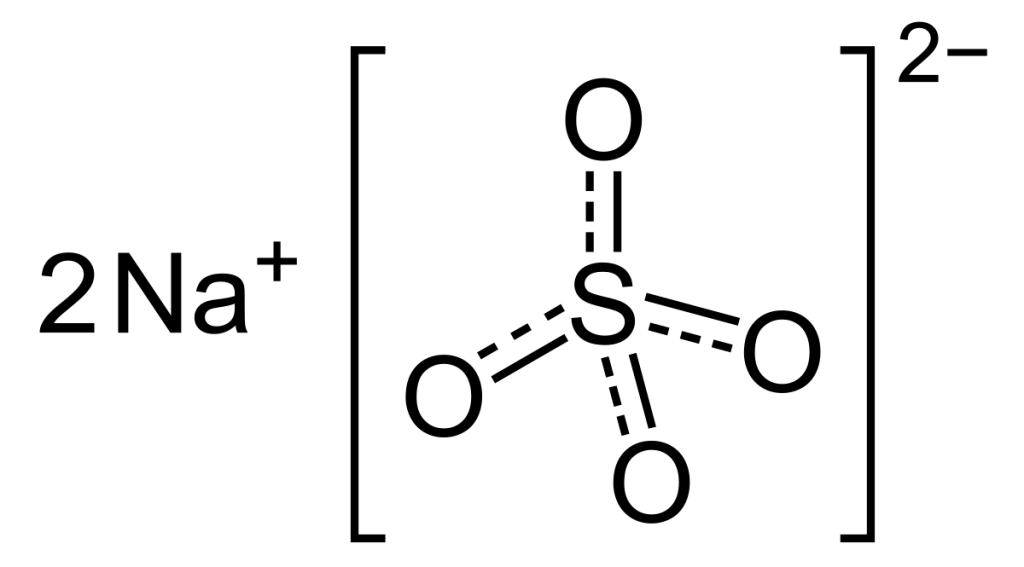
Physical Properties of Sodium Sulfate
| Appearance | White crystalline solid |
| Melting Point | 884C (Anhydrous); 32.38C (Decahydrate) |
| Boiling Point | 1429C (Anhydrous) |
| Solubility | Insoluble in ethanol; Soluble in water, Glycerol and hydrogen iodide |
| Refractive Index | 1.468 (Anhydrous); 1.394 (Decahydrate) |
| Density | 2.664 g/ml (Anhydrous); 1.464 g/ml (Decahydrate) |
| Odour | Odourless |

Chemical Properties of Sodium Sulfate
- It completely dissociates upon dissolving in water. This statement can be proved using a double displacement reaction.
Na2SO4 + BaCl2 -> 2NaCl + BaSO4
- This chemical does not react with most of the oxidizing or reducing agents. It can be converted into sodium sulfide by the method of carbothermal reduction.
Na2SO4 + 2C -> Na2S + 2CO2
- It reacts with sulfuric acid and forms sodium bisulfate, an acidic salt.
Na2SO4 + H2SO4 -> 2NaHSO4
Applications
- Glauber’s salt is used for tanning, dyeing, and for medicinal purposes.
- Glauber’s salt is also used in detergents and in the kraft process for paper pulping.
- Na2SO4 is used in organic reactions as a drying agent.
- It is also used as a fining agent in glass works to remove small air bubbles.
- Glauber’s salt is used as a laxative to remove certain drugs from the body.
- It is also used in defrosting windows, in carpet fresheners and also in starch manufacture.
FAQs
The solubility of anhydrous sodium sulfate at various temperatures:
1. At 0C, it is 4.76 g/100ml.
2. At 25C, it is 28.1 g/100ml.
3. At 100C, it is 42.7 g/100ml.
The solubility sharply increases when water is heater to 25C, and the same happens when it is again heated to 100C.
The names of this chemical vary with the water of crystallization present in them.
A. Names of Na2SO4.10H2O:
1. Glauber’s salt
2. Decahydrate sodium sulfate
3. Sal mirabilis
4. Mirabilite
B. Names of Na2SO4.7H2O:
1. Heptahydrate sodium sulfate
C. Names of Na2SO4:
1. Anhydrous sodium sulfate
2. Thenardite
The two important preparation processes of this chemical are:
1. The Mannheim Process:
H2SO4 + 2NaCl -> Na2SO4 + 2HCl
Here sulphuric acid and common salt are used to produce anhydrous sodium sulfate.
2. The Hargreaves Process:
4NaCl + 2H2O + 2SO2 + O2 -> 2Na2SO4 + 4HCl
Here common salt, water, sulphur dioxide and oxygen are used to produce this chemical.
It is not a toxic chemical but its dust causes eye irritation and also temporary asthma. These can be prevented by using a paper mask and eye protection gear while handling this chemical. There are no transport restriction for this chemical.
More on Inorganic Compounds
