Sodium Potassium Tartrate Tetrahydrate or Rochelle salt is a double salt of tartaric acid first prepared by an apothecary, Pierre Seignette, in about 1675.

| IUPAC Name | Sodium potassium-2,3-dihydroxy butane-1,4-dioate |
| Other Names | E337; Seignette’s salt; Rochelle salt |
| Chemical Formula | \(C_4H_4O_6KNa . 4H_2O\) |
Index
History
Pierre Seignette of La Rochelle, France, in about 1675, first prepared Potassium sodium tartrate tetrahydrate.
Piezoelectricity was first exhibited by Rochell’s salt and monopotassium phosphate. This property led to its extensive use in microphones and earpieces during post-World War II.
Structure of Sodium Potassium Tartrate
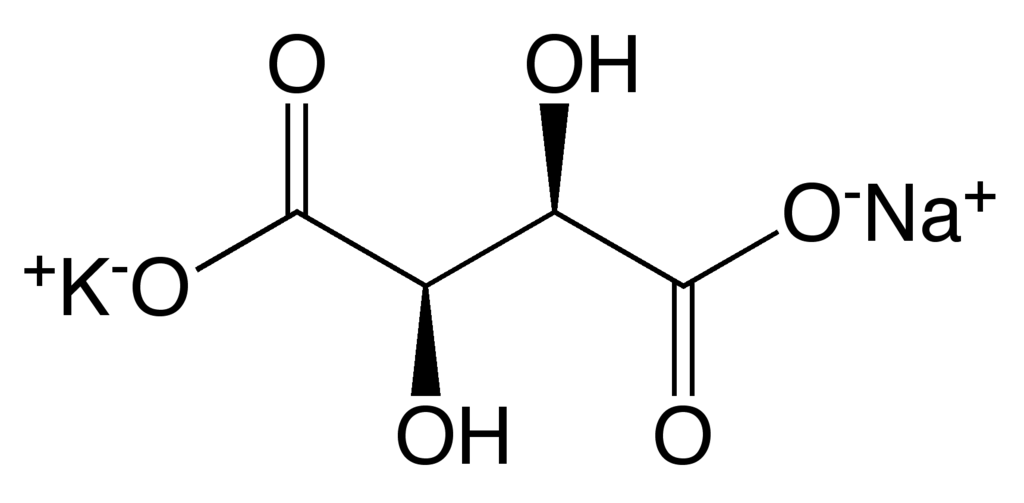
The sodium potassium tartrate is coordinated to ten oxygen atoms, four carbon atoms, one potassium, one sodium, and twelve hydrogen atoms.
This is a tetrahydrate, i.e. it has four water molecules (\(H_2O\)) attached to the salt.
Physical Properties
| Molecular Weight (Tetrahydrous) | 282.10 g/mol |
| Molecular Weight (Anhydrous) | 210.16 g/mol |
| Density | 1.79 g / cm3 |
| Melting Point | 75°C |
| Boiling Point | 220°C |
| Appearence | Large colorless monoclinic needles |
| Odour | Odorless |
| Water Solubility | 66g / 100ml (26°C) |
| Crystal Structure | Orthorhombic |
Chemical Properties
| Flammability | Non flammable or Combustible |
| Toxicity | Mildly toxic(For humans) |
| pH value | 7 – 8.5 |
Prepration
First step is conversion of Sodium bicarbonate to Sodium carbonate.
\(2NaHCO_3 \rightarrow Na_2CO_3 + CO_2 + H_2O\)
Potassium bitartrate reacts with sodium carbonate to generate the Rochelle salt.
\(KHC_4H_4O_6+ Na_2CO_3 \rightarrow C_4H_4O_6KNa . 4H_2O\)
Reactions
Fehling’s Solution Test
The reaction between the copper(II) ions and aldehyde in Fehling’s solution is written as:
\(RCHO + 2 Cu^{2+} + 5 OH^{-} \rightarrow RCOO^{-} + Cu_2O + 3 H_2O\)
or with the tartrate included:
\(RCHO + 2 Cu(C_4H_4O_6)_2 ^{2-} + 5 OH^{-} \rightarrow RCOO^{-} + Cu_2O + 4 C_4H_4O_6 ^{2-} + 3 H_2O\)
Applications
- Used as a Laboratory reagent.
- One of the ingredients in the Biuret reagent, to measure the protein concentration.
- Used in the process of electroplating (Increases cathode efficiency.)
- It helps in maintaining alkaline pH.
- It is an ingredient in Fehling’s solution test and determination of uric acid.
FAQs
Chemical formula of sodium potassium tartarate(tetrahydrated) is \(C_4H_4O_6KNa . 4H_2O\)
Rochell’s salt is another name for the chemical Sodium potassium tartarate(tetrahydrate), as it was first synthesized by Pierre Seignette of La Rochelle in 1675.
Rochell’s Salt is partially soluble in alcohols.
If consumed it may cause irritation, mildly hazardous.
More on Inorganic Compounds
