Barium Iodide is an inorganic compound with the chemical formula \(BaI_2\). The compound exists as an anhydrous and a dihydrate (\(BaI_2 \cdot (H_2O)\)) form.
Index
Structure and Formula
The IUPAC name of this inorganic compound is Barium iodide whose formula is \(BaI_2\) (anhydrous) & \(BaI_2 . 2H_2O\) (dihydrated).
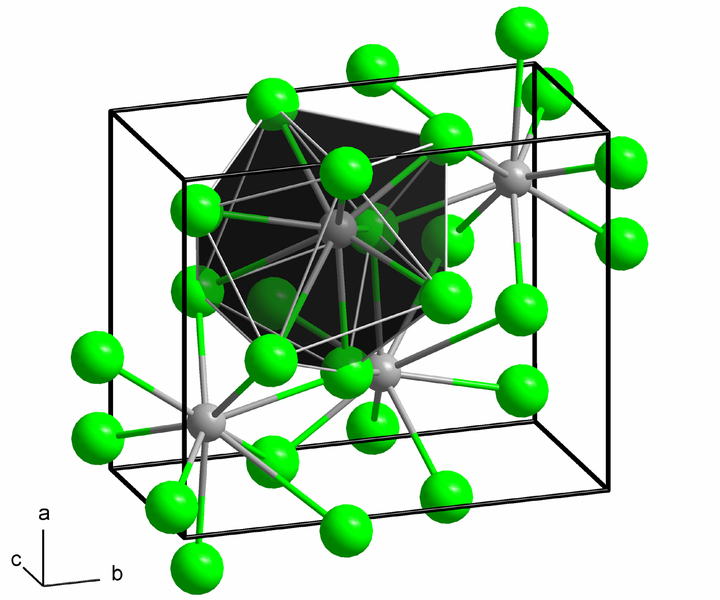
The structure of the anhydrous form is Orthorhombic, with each Ba centre bound to nine iodide ligands and has a crystalline packing structure.
Physical Properties
| Molecular weight (dihydrous) | 427.167 g/mol |
| Molecular weight(anhydrous) | 391.136 g/mol |
| Density | 1.79 g / cm3 |
| Melting point | 711°C (anhydrous) |
| Decompostion | 740°C (dihydrate) |
| Appearence | White Orthorhombic crystals |
| Odour | Odorless |
| Water solubility | 221g / 100ml (20°C) |
| Solubility | Soluble in ethanol, acetone |
| Crystal structure | Orthorhombic |
Chemical Properties
| Flammability | Non flammable |
| Toxicity | Toxic for humans |
| pH | 7 |
| Standard enthalpy of formation | -602.1 kJ . mol-1 |
Preparation
It can be made by the reaction of barium and iodine molecules as metals and halogens are highly reactive.
\(Ba + I_2 \rightarrow BaI_2\)
The reaction of potassium iodide with barium nitrate in the presence of water will yield dihydrate BaI2.
\(KI (aq) + Ba(NO_3)_2 (aq) \rightarrow KNO_3 (aq) + BaI_2(aq)\)
(This a double displacement reaction and is reversible)
Reactions
Barium iodide reacts with Potassium Bromide giving a yellow color precipitate.
\(KBr + BaI_2 \rightarrow KI(yellow ppt.) + BaBr_2\)
Barium sulfate formation,
\(BaI_2 + Na_2SO_4 \rightarrow BaSO_4 + 2NaI\)
Applications
- It is used in the preparation of iodide compounds (has no direct medical use).
- It is also used for the preparation of barium dioxide and other important compounds of Iodine.
Hazards
Like all other soluble salts of barium, BaI2 is also this hazardous and toxic.
FAQs
The formula for Barium Iodide(anhydrous) is \(BaI_2\) and Barium Iodide(dihydrous) is \(BaI_2\cdot 2(H_2O)\)
It is an ionic compound.
It is silvery gray; with a pale yellow tint.
More on Inorganic Compounds
| Mohr’s Salt (Fe(SO4)(NH4)2(SO4)(H2O)6) | Sodium Potassium Tartrate (C4H4O6KNa.4H2O) |
| Chlorine Trifluoride (ClF3) | Sulfur Trioxide (SO3) |
| Sodium Sulfate (Na2SO4) | Ammonium Bicarbonate ((NH4)HCO3) |
Other Salts Of Barium
