Sulfur Trioxide (SO3) is one of the most important oxides of sulfur, as it is used in the manufacture of sulfuric acid. It is often called “the most economically important sulfur oxide”. It is also prepared on a large scale, mainly for the production of sulfuric acid.

Index
Structure and Formula
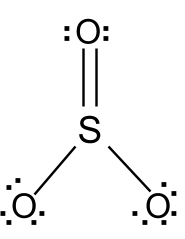
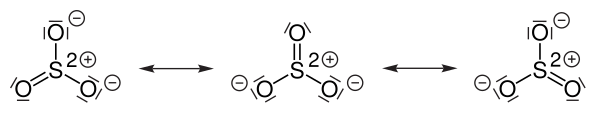
The chemical formula of sulfur trioxide is SO3. Gaseous monomer, crystalline trimer and solid polymer are some of the forms in which sulfur trioxide is found in nature. The shape of SO3 is triangular planar according to the VSEPR theory and the double bonds between S and O are dislocated due to which all the S-O bond lengths are equal.
Solid SO3 has 3 different crystal structures depending on the amount of water traces, and SO3 is equilibrium with its monomer and cyclic trimer forms in both its liquid and gaseous forms.
If SO3 is condensed at above 27°C then α-SO3 is formed, which is fibrous in appearance and melts at 62.3°C. β-SO3 is also similar to the α-SO3 as they both are fibrous, but their molecular masses are different as are their melting points, β-SO3 melts at 32.5°C.
Both γ-SO3 and β-SO3 slowly convert into α-SO3 as they both are metastable, this is caused by the traces of water.
The heating of a crystal of α-SO3 to its melting point can cause the glass container(in which the chemical is being heated) to break due to the sudden increase in vapour pressure, this phenomenon is called alpha explosion.
The cause of alpha explosion is, difference in the vapour pressures of the 3 types of SO3, their order is α < β < γ at the same temperature.
Physical Properties
| Sulfur Trioxide Molar mass | 80.066 g/mol |
| Appearance | Colourless or white solid and colourless liquid and gas |
| Density | 1.92 g/cm3(liquid) |
| Melting point | 16.9°C |
| Boiling point | 45°C |
| Solubility | Forms sulfuric acid upon dissolving |
| Standard entropy of formation \((\Delta_f H^{\theta}_{298})\) | -395.7 kJ/mol |
| Odor | Pungent(vapour); Odorless(mist) |
Chemical Properties
1. Hydration: This is one of the important reactions of sulfur trioxide.
\(SO_3 + H_2O \rightarrow H_2SO_4\)
Due to the vigorous fuming of gaseous sulfur trioxide, the freshly formed sulfuric acid is in the form of mist.
2. Hydrofluorination: This is the same as hydration, but instead of water HF is added to SO3 which forms fluorosulfuric acid.
\(SO_3 + HF \rightarrow FSO_3H\)
3. Deoxygenation: SO3 reacts with N2O5 to form a pyrosulfate of nitronium salt.
\(2SO_3 + N_2O_5 \rightarrow (NO_2)_2S_2O_7\)
4. Oxidation: It is a strong oxidant and so oxidizes compounds like sulfur chloride to thionyl chloride.
\(SO_3 + SCl_2 \rightarrow SOCl_2 + SO_2\)
5. Sulfur trioxide reacts with sodium hydroxide to form sodium hydrogen sulphate.
\(SO_3 + NaOH \rightarrow NaHSO_4\)
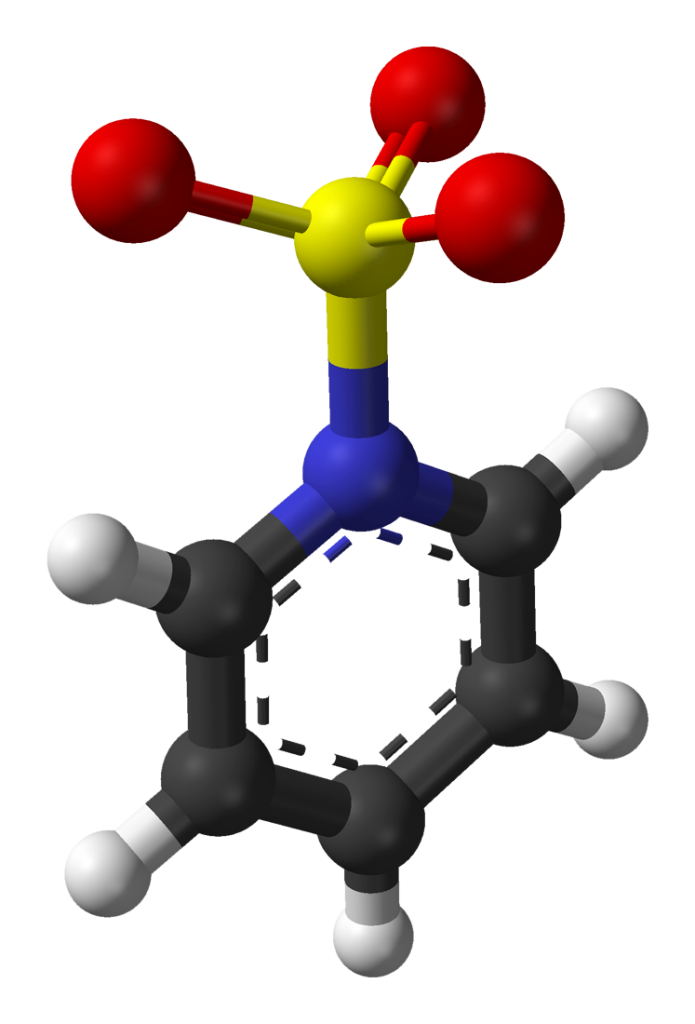
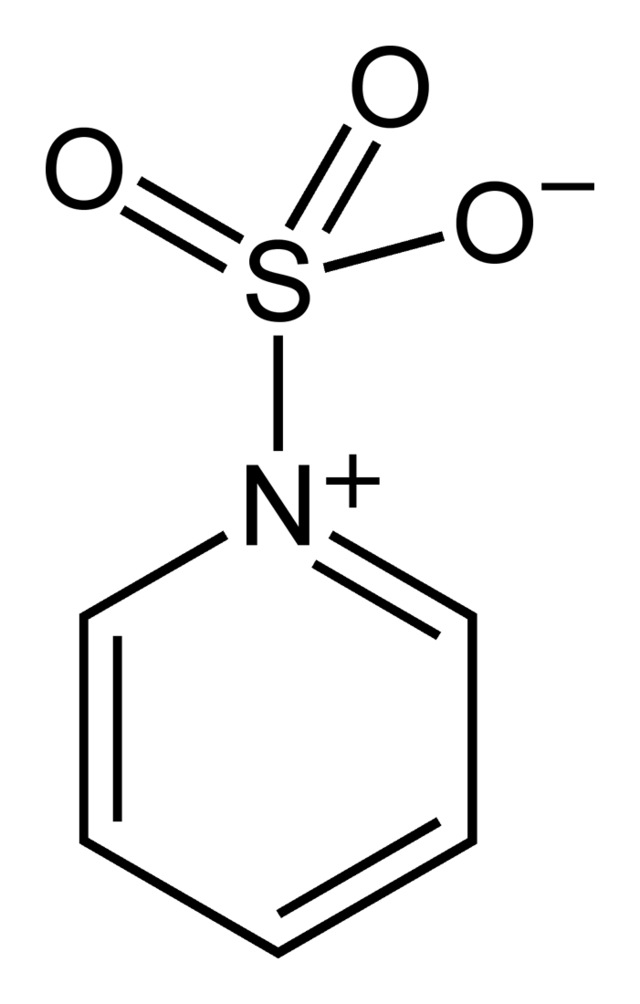
6. Lewis Acid: Due to the electrophilic nature of SO3 it is also a strong lewis acid and readily reacts with lewis bases, like pyridine with which it forms a complex, the same happens with dioxane and trimethylamine.
Production of Sulfur Trioxide
1. The direct oxidation of SO2 to produce SO3 is possible and even occurs in nature, but this is a very slow process which makes it less feasible commercially.
\(SO_2 + \frac{1}{2}O_2 \rightarrow SO_3 ; \Delta H = -198.4\)
2. SO3 can be synthesized by the pyrolysis of sodium hydrogen sulphate or sodium bisulfate.
- Dehydration at 315°C:
\(2NaHSO_4 \rightarrow Na_2S_2O_7 \mbox{ (Sodium Pyrosulphate) } + H_2O\) - Cracking at 460°C:
\(Na_2S_2O_7 \rightarrow Na_2SO_4 + SO_3\)
The dehydration of sulfuric acid with P4O10 (Phosphorus Pentoxide). The above two methods of production are usually used in laboratories.
3. The commercial production of sulfur trioxide is through the contact process, for which sulfur dioxide is produced from either the burning of sulfur or iron pyrite.
The speed of oxidation of sulfur dioxide is increased by adjusting the reaction conditions like temperature which is set between 400°C and 600°C and catalysts like vanadium pentoxide are also used. Platinum can also be used as a catalyst, but it is not as it is costly and is poisoned easily by impurities.
Applications of Sulfur Trioxide
- The coumpound is used in the production of pharmaceuticals, detergents and dyes, as it is used in sulfonation reactions.
- It is used in the production of sulfuric acid, by dissolving it in sulfuric acid and then diluting the formed oleum with water.
- Used to remove residual hydrogen peroxide.
- Used to separate pulp from lignin and acts as a digesting agent.
- The production of solar energy devices and photoelectric cells uses SO3.
Hazards of Sulfur Trioxide
- The compound can cause fire when it comes in contact with organic materials like wood and cotton.
- It can cause skin burns.
- Its fumes are highly toxic upon inhalation.
- Its ingestion causes severe burns in the mouth, food pipe and stomach.
- SO3 reacts violently with water to form even more corrosive sulfuric acid, sometimes in mist form.
- This is also largely responsible for acid rains.
When inhaled, remove the victims to fresh air immediately and check for breathing, then get medical assistance. When ingested first rinse the mouth thoroughly with water and this water must not be swallowed, do not induce vomiting, then give the victim milk or water, the quantity depends upon their age. If the eye is exposed to it then rinse it thoroughly with lukewarm water for a minimum of 15 min.
FAQs
The molar mass of SO3 = 80.066 g
The mass of sample SO3 = 78.0 g
Let, the no. of molecules of SO3 be \(n\)
No. of molecules in a sample of a compound of mass \(m\) and molar mass \(A\) is,
\(\mbox{No. of molecules($n$)} = \frac{\mbox{mass of the sample($m$)}}{\mbox{molar mass of the compound(A)}} \times (6.022 \times 10^{23})\)
So, using this formula we calculate the number of molecules of SO3.
Hence,
\(n = \frac{78.0}{80.066} \times 6.022 \times 10^{23}\)
\(n = 5.458 \times 10^{23}\)
So, the number of molecules in 78 grams is \(5.458 \times 10^{23}\).
The formula of Sulfur Trioxide is SO3.
Sulfuric acid is not manufactured by directly adding sulfur trioxide to water. It is produced by the absorption of sulfur trioxide into sulfuric acid.
This happens when sulfuric acid is concentrated with SO3 to form oleum, which is then diluted using water to form sulfuric acid again.
The central sulfur in sulfur trioxide is partially positively charged due to the relatively very high electronegativity of its surrounding oxygen atoms, which produces a partial positive charge on the sulfur. This results in the electrophilic nature of sulfur trioxide.
Sulfur trioxide is an electrophile, so it attracts electrons and negative charges towards it, due to this it can take electrons from a molecule thereby oxidizing it. Hence, sulfur trioxide is an oxidant.
More on Inorganic Compounds