Ammonium bicarbonate ((NH4)HCO3) is a white crystalline inorganic compound. It is a white powder-like salt and is soluble in water.
It is also occurs naturally as a very rare mineral known as “Teschemacherite”.
Index
Structure and Formula
Ammonium bicarbonate’s formula is (NH4)HCO3. It is an ionic compound with +1 charge on the cation and -1 charge on the anion. Its structure is as follows:
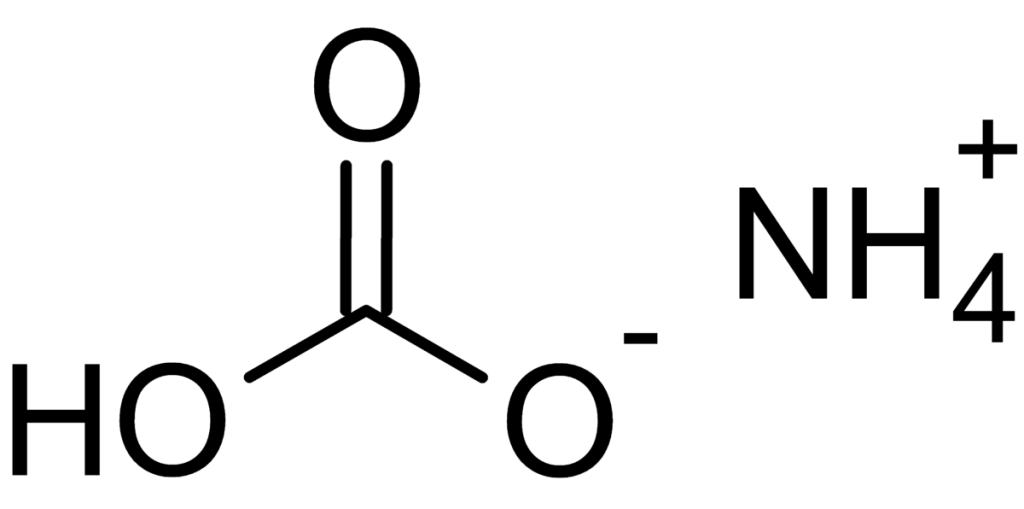
Molar mass of ammonium bicarbonate is 79.056 g/mol.
Physical Properties of Ammonium Bicarbonate
| Appearance | White, crystalline solid |
| Molar Mass | 79.056 g/mol |
| Density | 1.59 g/cm³ |
| Melting Point | 41.9 °C |
| Solubility | 24.8 g/100 mL (25 °C) in water. Insoluble in MeOH |
| Odour | Mild ammonia-like odour |
Chemical Properties
- It readily decomposes into carbon dioxide, water and ammonia (at about 36oC).
(NH4)HCO3 → NH3 + H2O + CO2
- (NH4)HCO3 on reaction with acids produces ammonium salts.
(NH4)HCO3 + HCl → NH4Cl + CO2 + H2O
Reaction with bases produces ammonia.
(NH4)HCO3 + 2 NaOH → Na2CO3 + NH3•H2O + H2O
- (NH4)HCO3 reacts with alkali metal halides to give alkali metal bicarbonate and ammonium halide.
(NH4)HCO3 + KCl → NH4Cl + KHCO3
(NH4)HCO3 + NaBr → NH4Br + NaHCO3
Production of Ammonium Bicarbonate
The compound can be prepared by passing carbon dioxide gas through a cold solution of ammonia water.
CO2 + NH3 + H2O ↔ (NH4)HCO3
Since ammonia bicarbonate is thermally unstable and can revert back to the constituents, the solution is kept cold to prevent this.
Applications of Ammonium Bicarbonate
Following are the applications of this white-crystalline compound:
- Application in food – it is used in baking cookies and crackers. It was used as baking powder in the past before being replaced by sodium bicarbonate in order to avoid ammonia traces in food.
- It is used in the manufacture of ceramics.
- Also used as a source of ammonia gas.
- It is sometimes used as a basic, cheap fertilizer. It is being replaced by urea due to better thermal stability.
Ammonium Bicarbonate Hazards
This inorganic compound is an irritant. Inhaling dust containing (NH4)HCO3 can irritate the lungs and cause bronchitis with cough and shortness of breath. High levels of exposure can result in these symptoms lasting several months or even years.
Coming in contact with (NH4)HCO3 can irritate eyes and cause skin allergies.
FAQs
Ammonium Bicarbonate is an inorganic compound that is a white crystalline solid. It is soluble in water and is used in food manufacture, fire extinguishers and dye production. Its formula is (NH4)HCO3.
It (NH4)HCO3 is prepared by passing carbon dioxide gas through a cold solution of ammonia water.
CO2 + NH3 + H2O ↔ (NH4)HCO3
Since the compound is thermally unstable and can revert back to the constituents, the solution is kept cold to prevent this.
Its molar mass is 79.056 grams per mole.
More Inorganic Compounds
