Mohr’s Salt (Fe(SO4)(NH4)2(SO4)(H2O)6) named after the German scientist Karl Friedrich Mohr, is an inorganic compound also known as ammonium iron(II) sulfate or ferrous ammonium sulphate. This compound contains two primary cations, the ammonium cation(NH4)+ and the ferrous cation(Fe2+).
Index
Structure & Formula
The chemical formula of Mohr’s salt (hexahydrate) is Fe(SO4)(NH4)2(SO4).6H2O and anhydrous form is Fe(SO4)(NH4)2(SO4).
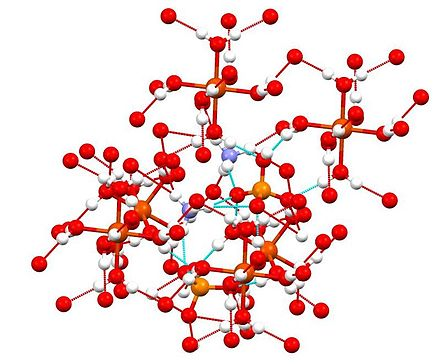
The inorganic compound is a member of a group of double sulfates known as Schonites or Tutton’s salts. All members of this group form crystals with a monoclinic geometry.
The bonding patterns of the compound show octahedral centres made of {Fe(H2O)6]2+ centres. These centres are known to form hydrogen bonds with the ammonium sulfate ions.
Physical & Chemical Properties
| Appearance | Blue-green solid |
| Molar mass | 284.05 g mol-1(anhydrous)392.13 g mol-1(hexahydrate) |
| Density | 1.86 g/cm3 |
| Melting point | 100 to 110 °C (212 to 230 °F; 373 to 383 K) |
| Boiling point | NA |
| Solubility | Soluble in water, insoluble in ethanol |
| Odor | Weak ammonia-like |
Preparation of Mohr’s Salt
Preparation of Mohr’s salt typically involves the dissolution of ammonium sulfate and hydrated ferrous sulfate in water that contains a small amount of sulfuric acid. Ammonium sulfate and hydrated ferrous sulfate are mixed in an equimolar ratio.
A solution is obtained as a result which is subjected to a crystallization process in order to obtain light green crystals of the salt.
Dilute sulfuric acid is added in order to prevent the hydrolysis of ferrous sulfate. During dissolution, excessive heating is generally avoided. This prevents the conversion of Fe+2 ions(that are light green in colour) to Fe3+ ions(yellow in colour).
If a yellow solution is obtained, the process must be repeated. If the crystals do not separate upon cooling, it is recommended to add a few crystals of Mohr’s salt to the concentrated solution in order to promote crystal growth, which is also known as seeding.
Applications of Mohr’s Salt
- In analytical chemistry, the compound is the preferred source of ferrous ions due to the long shell life of the solid.
- It is used in Fricke’s dosimeter in order to measure high doses of gamma rays.
- It is used in common labs for performing a qualitative chemical analysis, which is used for the identification of the unknown concentration of a given solution.
Hazards
The inorganic compound may cause eye, skin, and respiratory tract irritation. Ingestion of this salt causes gastrointestinal irritation with nausea, vomiting, and diarrhea. It may also cause chronic liver damage.
FAQs
Mohr’s salt is an inorganic compound with the chemical formula Fe(SO4)(NH4)2(SO4).6H2O also known as ferrous ammonium sulphate.
It is used in labs for the identification of the unknown concentration of a given solution, in Fricke’s dosimeter to measure high doses of gamma rays and is also a preferred source of ferrous ions.
Mohr’s salt is also called a double salt which contains more than one simple salt due to the presence of two primary cations, the ammonium cation, and the ferrous cation.
More on Inorganic Compounds
