Potassium Dichromate is a popular inorganic oxidizing agent in both industries and laboratories. Its chemical formula is K2Cr2O7. It is largely used in laboratories as it is not a deliquescent salt. It is crystalline orange coloured salt and is harmful to health upon consumption.

Index
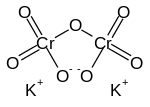
Structure and Formula
Potassium dichromate is bonded by an ionic bond between potassium(K) and dichromate ion. The shape of the hybrid Cr orbital in dichromate ion is tetrahedral and its hybridization is sp3. The crystal of the compound is of triclinic form below 241.6°C.
Physical Properties
| Potassium Dichromate Molar Mass | 294.185 g/mol |
| Appearance | Orange crystalline solid |
| Density | 2.676 g/cm3 |
| Melting Point | 398°C (Polymorphic Transformation) |
| Boiling Point | 500°C, Starts decomposing from 1360°C |
| Solubility | Insoluble in alcohol and acetone; Solubility in water increases with increase in temperature |
| Heat Capacity | 219 J/mol.K |
| Odour | Odourless |
| Refractive Index | 1.738 |
Chemical Properties
- Oxidation Reactions
- The compound is a mild oxidizing agent compared to potassium permanganate(KMnO4), so it is used in the production of aldehydes from alcohols.
- It converts secondary alcohols into ketones whereas tertiary alcohols cannot be oxidized.
- After reaction with an alcohol the solution changes colour from orange to green, this is due to the reduction of the dichromate ion from +6 oxidation state to +3. This color change is used as a test for aldehydes and ketones, as when ketones are formed there is no colour change in the solution.
- Potassium dichromate oxidizes ferrous salts to ferric salts.
K2Cr2O7 + 7H2SO4 + 6FeSO4 -> K2SO4 + Cr2(SO4)3 + 3Fe2(SO4)3 + 2H2O
- Upon Heating
- The compound decomposes and releases oxygen upon strong heating.
4K2Cr2O7 -> 4K2CrO4 + 2Cr2O3 + 3O2
- The compound decomposes and releases oxygen upon strong heating.
- Reaction with Alkali
- When K2Cr2O7 reacts with an alkali the solution changes colour from orange to yellow due to the formation of chromate ion (CrO42-).
K2Cr2O7 + K2CO3 -> 2K2CrO4 + CO2
- When K2Cr2O7 reacts with an alkali the solution changes colour from orange to yellow due to the formation of chromate ion (CrO42-).
- Reactions with Sulfuric Acid
- Reaction of potassium dichromate and cold sulfuric acid gives red coloured crystals of chromic anhydride (CrO3).
K2Cr2O7 + 2H2SO4 -> 2CrO3 + 2KHSO4 + H2O - When K2Cr2O7 is heated along with concentrated sulfuric acid it evolves oxygen.
2K2Cr2O7 + 8H2SO4 ->2K2SO4 + 2Cr2(SO4)3 + 8H2O + 3O2 - Chromyl Chloride Test
When a chloride containing salt reacts with a solution containing concentrated sulfuric acid and potassium dichromate reddish brown vapour of chromyl chloride is evolved. This reaction is used in the test for chloride ions in salts.
K2Cr2O7 + 4KCl + 6H2SO4 -> 2CrO2Cl2 + 6KHSO4 + 3H2O
- Reaction of potassium dichromate and cold sulfuric acid gives red coloured crystals of chromic anhydride (CrO3).
Production of Potassium Dichromate
- The compound can be produced from the reaction of sodium dichromate and potassium chloride.
Na2Cr2O7 + 2KCl -> K2Cr2O7 + 2NaCl - It can also be produced from potassium chromate by the roasting of chromate ore with potassium hydroxide.
- The compound occurs naturally as the rare mineral Lopezite. It has only been found in the nitrate deposits of the Atacama desert in Chile.

Applications of Potassium Dichromate
- It is used to produce potassium chrome alum used in tanning leather.
- The compound can be used to prepare chromic acid for cleaning and etching glassware. But this method is not being used now-a-days due to the safety concerns.
- It is also used in cement to improve its texture and density and increase its setting time.
- It is used in the analysis of purity of a sample of chemical:
- Concentration of ethanol in a solution by back titration using acidified potassium dichromate.
- Potassium dichromate in 35% nitric acid is schwerter’s solution, which is used in the analysis of purity of various metals, the major one being silver.
- Potassium dichromate paper is used to test for sulfur dioxide, as it changes colour from orange to green.
- It is used in staining wood into deep and various shades of brown which cannot be achieved by paints.
Hazards of Potassium Dichromate
- The compound is one of the most common causes of chromium dermatitis, it is a chronic disease and is difficult to treat when infected to the hand or fore-arm.
- It is a carcinogenic compound.
- Its highly corrosive in nature and can cause eye damage or even blindness when exposed to eyes. It can also cause burns to the exposed skin.
- Exposure to this compound can cause impaired fertility, heritable genetic damage and can cause harm to even unborn children.
FAQs
The formula for potassium dichromate is K2Cr2O7.
Some of the major health hazards of the compound:
1. Impaired fertility
2. Genetic damage which is heritable
3. Harm to unborn children
4. Carcinogenic
5. Highly corrosive
Potassium dichromate is a non-deliquescent chemical unlike sodium dichromate. So it is more often used in laboratories than sodium dichromate.
It occurs as the rare mineral lopezite in nature.
Know More on Inorganic Compounds
