Calcium nitrate is an inorganic compound. It is also known as Norwegian Saltpeter. It has the formula Ca(NO3)2 and is usually found as a tetrahydrate compound Ca(NO3)2.4H2O.
The inorganic compound was synthesized by the Birkeland Eyde process at Notodden, Norway, in 1905.
Index
Structure and Formula
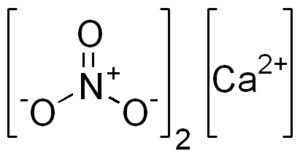
The chemical formula of calcium nitrate is Ca(NO3)2 and the structure is represented below.
Physical Properties
| Odor | No odor |
| Appearance | White deliquescent granules |
| Melting point | 561℃ |
| Boiling point | decomposes |
| Solubility | Soluble in water, ethanol, methanol, acetone |
Chemical Properties
Upon heating, the compound undergoes decomposition and releases nitrogen dioxide and oxygen.
Ca(NO3)2 →CaO + 2NO2 + 1/2O2
On adding calcium nitrate to sodium carbonate, a precipitate of calcium carbonate is formed, leaving sodium nitrate in the solution.
Ca(NO3)2 + Na2CO3 → 2NaNO3 + CaCO3
Preparation
The compound Ca(NO3)2 is prepared by the following reactions
It is obtained by the reaction of calcium carbonate (generally as limestone) with nitric acid:
CaCO3 + 2HNO3 ? Ca(NO3)2 + CO2 + H2O
It can be obtained in the extraction process of calcium phosphate as a byproduct:
Ca3(PO4)2 + 6HNO3 + 12H2O ? 2H3PO4 + 3Ca(NO3)2 + 12H2O
It is obtained by the reaction of ammonium nitrate solution and calcium hydroxide:
2NH4NO3 + Ca(OH)2 ? Ca(NO3)2 + 2NH4OH
Applications
- The inorganic compound is used in agriculture, in order to control certain plant diseases.
- Finds applications in waste-water treatment, for odor emission prevention.
- It is used in accelerating concrete admixtures.
- The tetrahydrate form is highly endothermic, which allows it to be used for regenerable cold packs.
- It is a very common coagulant in latex production.
- It can also be used as a part of molten salt mixtures, for the purpose of heat transfer and storage.
Hazards
- The inorganic compound is a powerful oxidizing agent and presents a dangerous fire hazard.
- It has an irritating effect on skin and mucous membranes.
FAQs
It is primarily used as a nitrogen fertilizer in agriculture.
It can cause headaches, dizziness, and nausea and being an oxidizing agent, it has a danger of fire hazard.
It is produced by applying nitric acid to limestone, and then adding ammonia.
More on Inorganic Compounds
