Sodium Dichromate is a bright orange inorganic chromium salt that is very similar to potassium dichromate in its appearance. Its chemical formula is Na2Cr2O7, but is used in its dihydrate form Na2Cr2O7.2H2O.
The compound is around 20 times more water-soluble than potassium dichromate, it also has a relatively low equivalent weight. The production of almost all chromium ores and chromate based compounds is through this salt.

Index
Structure and Formula
The bonding of this inorganic chromium salt is similar to that of potassium dichromate, as the anion is the same in both the compounds and the only cation is different, so it has an ionic bond between two Na+1 and one Cr2O72-. Dichromate ion is sp3 hybridised and has a tetrahedral structure.
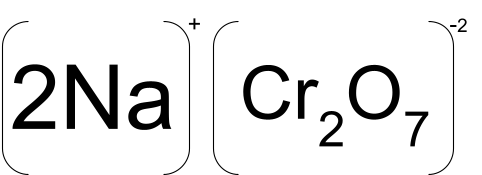
Physical Properties
| Molar Mass | 261.97 g/mol |
| Appearance | Bright orange crystalline solid |
| Density | 2.52 g/cm3 |
| Melting Point | 356.7°C (Polymorphic Transformation) |
| Boiling Point | 400°C, Starts decomposing from 1360°C |
| Solubility | Soluble in water, Methanol and Ethanol |
| Odour | Odourless |
| Refractive Index | 1.661 (Dihydrate) |
Chemical Properties
- Oxidation:
In organic synthesis, the compound is used to convert benzylic and allylic C-H bonds into carbonyl derivatives like aldehydes, ketones or carboxylic acids.
3R2CHOH + Cr2O72- + 2H+ -> 3R2C=O + Cr2O3 + 4H2O
- It is used in the conversion of fluorene to fluorenone.
Production of Sodium Dichromate
On an industrial scale, the compound is produced from chromium(III) oxide ores. It is produced by fusing with a base at 1000°C in the presence of air.
2Cr2O3 + 4Na2CO3 + 3O2 -> 4Na2CrO4 + 4CO2
In the above step, chromium is made to be extracted into hot water by solubilizing it. Then acidifying the resulting extract with sulfuric acid or carbon dioxide results in the formation of sodium dichromate.
2Na2CrO4 + 2CO2 + H2O -> Na2Cr2O7 + 2NaHCO3
2Na2CrO4 +H2SO4 -> Na2Cr2O7 + Na2SO4 + H2O
Then the resulting sodium dichromate is crystallized in its dihydrate form.
Applications of Sodium Dichromate
- The compound is used in the production of many other chromium products. But it is also used in the production of chromium sulfate.
- It is used in the tanning of leather.
- It is used to prevent the corrosion of metallic surfaces and also lets paint stick to them.
- Used in the production of wax and vitamin K, as an oxidiser.
- Also used in colour glass production and also in ceramic glaze.
Hazards of Sodium Dichromate
- Inhaling the dust or mist of this inorganic salt can lead to respiratory problems resembling asthma and nasal septal perforation.
- Exposure to eyes and skin lead to dermatitis and local irritation.
- It is highly corrosive.
- Its consumption can lead to diarrhoea and vomiting.
- It is a carcinogenic compound.
- The compound is a non-combustible compound, but it can support the combustion of other combustible compounds and can explode and catch fire when it comes in contact with such compounds.
FAQs
The chemical formula for sodium dichromate is Na2Cr2O7.
The molar mass of the compound is 261.97 g/mol.
Bichromate of soda, disodium dichromate and sodium dichromate(VI) are the other common names of this inorganic salt.
It is first reduced with sulfur dioxide for the tanning of leather.
More on Inorganic Compounds
