Chromic Acid is the mixture of concentrated sulfuric acid and a dichromate ion, which may contain a variety of compounds. It is also known as Tetraoxochromic acid or Chromic(VI) acid.
Index
More About Chromic Acid
There are two forms of chromic acids:
- Molecular Chromic Acid (H2CrO4): Similar to H2SO4.
- Dichromic Acid (H2Cr2O7): A fully protonated form of the dichromate ion(Cr2O7–).
Following are the properties of the chemical
| Chemical Formula | H2CrO4 or H2Cr2O7 |
| Molecular Weight | 118.008 g/mol |
| Density | 1.201 g/cm3 |
| Melting Point | 197℃ |
| Boiling Point | 250℃ |
Chromic Acid Test for Alcohols and Aldehydes
Now, let’s have a look at this test for alcohols and aldehydes which involves this chemical (Oxidation Mechanism)
Procedure:
Three drops of the unknown compound to be tested are mixed with 5 drops of acetone and 5 drops of tetraoxochromic acid solution.
Result:
The formation of a bluish-green colour of the Cr(III) ion indicates a positive test. If the unknown compound is insoluble in water, two layers are present. A bluish-green colour in either layer indicates a positive test.
Reactions:
Aldehydes and primary alcohols are oxidized to carboxylic acids while the Cr+6 ion in the tetraoxochromic acid is reduced to Cr+3 ion.
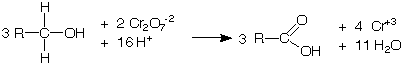
Secondary alcohols are oxidized to ketones while the Cr+6 ion in the tetraoxochromic acid is reduced to Cr+3 ion.
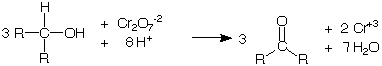
Applications of Chromic Acid
- Used in chromium plating.
- Used in ceramic glazes and colored glass.
- Can be used to clean laboratory glassware.
- Used in hair dye.
FAQs
The formula is H2CrO4(molecular form) and H2Cr2O7(dichromic form).
It is the mixture of a concentrated sulfuric acid and dichromate ion.
Sodium dichromate or potassium dichromate must be first combined with water to produce a paste. Continuous addition of concentrated sulfuric acid and mixing produces tetraoxochromic acid.
More on Inorganic Compounds
