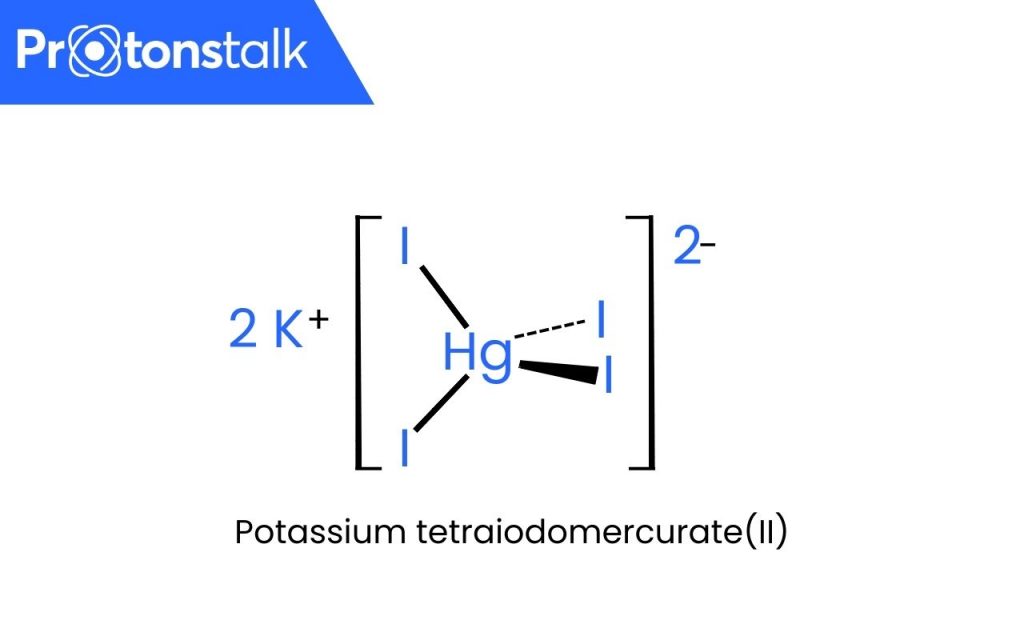
The Nessler reagent, or potassium tetraiodomercurate, is composed of potassium cations and an anion called tetraiodomercurate (II).
Its molecular formula can be represented as [K2(HgI4)].
Julius Nessler, a German chemist, created a chemical solution known as Nessler’s reagent in the late 19th century, which can be used to measure the concentration of ammonia by analyzing its colour.
Index
Explanation
Nessler Reagents [K2(HgI4)] primary purpose is to detect the presence of ammonia and ammonium compounds in a sample.
To prepare Nessler Reagent, sodium hydroxide and potassium iodide are mixed with mercuric chloride or mercury iodide.
The molecular formula of Nessler’s reagent is [K2(HgI4)].
Preparation Of Nessler’s Reagent
The preparation of Nessler’s reagent can be done by following steps:
- Measure out 2 grams of potassium iodide and add it to a clean container.
- Add 5 milliliters of water to the container with the potassium iodide.
- Stir the solution until the potassium iodide is completely dissolved.
- Measure out 3 grams of mercury (II) iodide and add it to the solution of potassium iodide and water.
- Stir the solution until the mercury (II) iodide is completely dissolved.
- Add enough water to bring the total volume of the solution up to 20 milliliters.
- Measure out 40 grams of 30% potassium hydroxide.
- Add the potassium hydroxide to the solution of mercury (II) iodide and potassium iodide.
- Stir the solution until the potassium hydroxide is completely dissolved.
The resulting solution is Nessler’s reagent, which should be pale orange in color.
Nessler’s Reagent Reaction
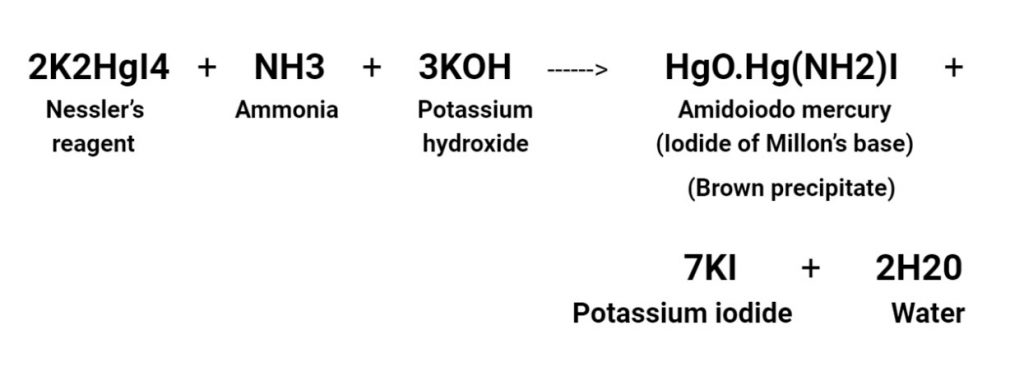
The reagent turns to a dark shade of yellow when exposed to ammonia.
If the concentration of the solution is high, which may result in the formation of a precipitate with a brown colour.
The brown precipitate, which was formed is a derivative of Millon’s base, can be expressed in two formulas: 3HgO·Hg(NH3)2I2 and NH2·Hg2I3.
Applications
- Nessler’s reagent is used to detect the presence of ammonia, which causes the solution to turn a deep yellow color, eventually resulting in the formation of a brown precipitate.
- Nessler’s reagent is also used for the qualitative analysis of ammonia.
FAQ’S
Nessler’s reagent, or potassium tetraiodomercurate, is an inorganic compound, which is an alkaline solution of K2HgI4 (potassium tetraiodomercurate) which is composed of potassium cations and an anion called tetraiodomercurate (II).
Potassium tetraiodomercurate(II)
Nessler’s reagent is used to detect trace amounts of ammonia.
Nessler’s Reagent is a solution utilized for identifying the existence of ammonia and ammonia salts, which initially have a pale orange colour.
K2(HgI4) is the molecular formula of Nessler’s reagent.
Related topics
