Schiff reagent(C19H21N3S2O7.4H2O) is a compound that results from the reaction between sodium bisulfite and fuchsin(C20H19N3.HCl), a magenta-coloured dye.
It is commonly used as a chemical test to identify the presence of aldehydes in a sample as aldehydes can react with the decolourized Schiff reagent and restore their original colour.
German chemist Hugo Schiff discovered Schiff Reagent. The first known use of Schiff’s reagent was in 1897.
In 1924, Feulgen utilized Schiff’s reagent for the first time in biological research. The staining method was subsequently named after him.
Feulgen employed Schiff’s reagent to identify aldehydes produced by the acidic hydrolysis of deoxyribose sugar in deoxyribonucleic acid (DNA).
Index
Explanation:
The schiff reagent is a compound that results from the reaction between sodium bisulfite and fuchsin, a magenta-coloured dye.
This reagent is useful in detecting the presence of aldehydes in a sample and can differentiate between aldehydes and ketones.
Fuchsine is decolourized by sulphur dioxide, and this property is utilized in the formation of Schiff reagents.
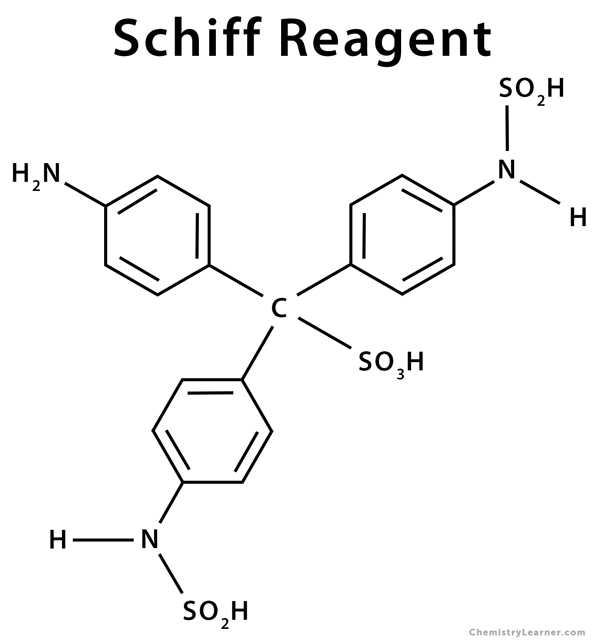
Composition
Schiff reagent formula: C19H21N3S2O7.4H2O
Composition
Basic Fuchsin Hydrochloride (C20H19N3.HCl): <1%
Hydrochloric Acid (HCl): <1%
Sodium bisulfite (NaHSO3): <1%
Water (H2O): >98%
The method involves shaking the solution at fixed intervals and then using sulphurous acid (or its conjugate base bisulphate) for decolourization of the solution and then adding charcoal. It is important to use charcoal to guarantee a completely colourless solution. If the solution still contains colour, it should be filtered again.
Schiff Reagent Preparation
Following is the method of preparation of the reagent
- Dissolve 5g of basic fuchsin in 900 ml of boiling water.
- Allow the solution to cool to around 50 degrees Celsius.
- Gradually add 100 ml of 1M HCl to the diluted fuchsin solution.
- Reduce the temperature of the solution to 25 degrees Celsius.
- Add 10g of NaHSO3 to the cooled solution.
- Shake the solution for 3 minutes and then incubate it in a dark place for 24 hours.
- After incubation, add 5g of fine activated charcoal to the mixture.
- Filter the solution after shaking for 3 minutes.
- Repeat the filtration and treatment process if a clear solution is not obtained.
- Store the prepared solution in a foil-covered vial at 4 degrees celsius to prevent the precipitation of white crystalline material.
- It is recommended to prepare fresh batches of the Schiff Reagent every 2-3 weeks to ensure accurate results.
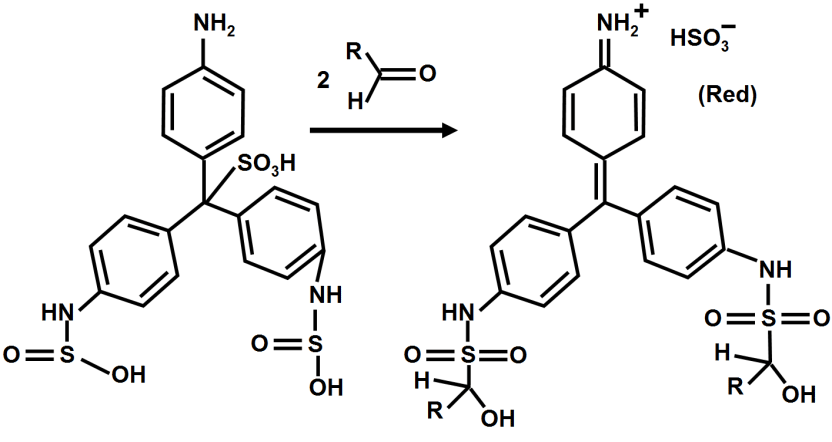
Mechanism:
Following is the mechanism of the Schiff test.
The reagent is a colourless adduct with a sulfonated central carbon produced by the combination of bisulfite and pararosaniline.
Any aldehyde group would then react with the uncharged amine groups from the aromatic ring to create an aldimine group.
This aldimine group is an electrophile, which pushed it into further reaction with bisulfite ion.
Finally, this results in the formation of a purple or magenta color solution.
Applications
- Schiff Reagent is used to check for the presence of aldehydes in a given analyte.
- It is also used to distinguish between aldehydes and ketones.
FAQ’S
Schiff’s reagent gives pink colour on reaction with Acetaldehyde
The Schiff’s reagent will change colour from colourless to magenta once an aldehyde(containing alpha hydrogen) is introduced.
p-rosaniline hydrochloride solution also known as Schiff Reagent is used in Schiff tests.
The schiff reagent is a reagent used in Schiff’s test for aldehydes.
Related Topics
