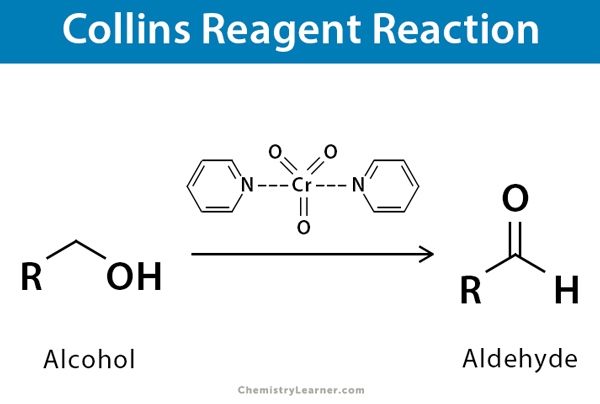
Collin’s reagent, which consists of dipyridine-complex chromium(VI) oxide in dichloromethane, is utilized to specifically transform primary alcohols into aldehydes through oxidation.
It can accommodate various functional groups present in the molecule.
Collins reagent formula is C10H10CrN2O3.
In 1953, G. I. Poos, G. E. Arth, R. E. Beyler, and L.H. Sarett discovered the use of this reagent for oxidations, which was later promoted by J.C. Collins after a few years.
In 1968, J.C. Collins introduced the concept of Collins oxidation, wherein he utilized a solution of pre-formed CrO3•2Pyr in dichloromethane to bring about the oxidation of alcohols.
Index
Explanation
Collins reagent, which is a red-coloured dipyridine complex composed of chromium (VI) oxide in dichloromethane, is employed for the oxidation of primary alcohols to aldehydes, without over-oxidation.
It has the molecular formula C10H10CrN2O3.
The process of utilizing Collins reagent for the oxidation process is referred to as Collins oxidation.
Furthermore, it can serve as a substitute for Jones reagent, Sarett Reagent, and pyridinium chlorochromate (PCC) for oxidizing secondary alcohols to ketones.
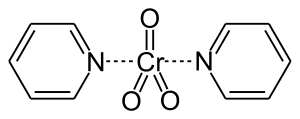
Structure of Collins Reagent (Source)
Reaction
Following image shows the collins reagent reaction converting a alcohol into aldehyde
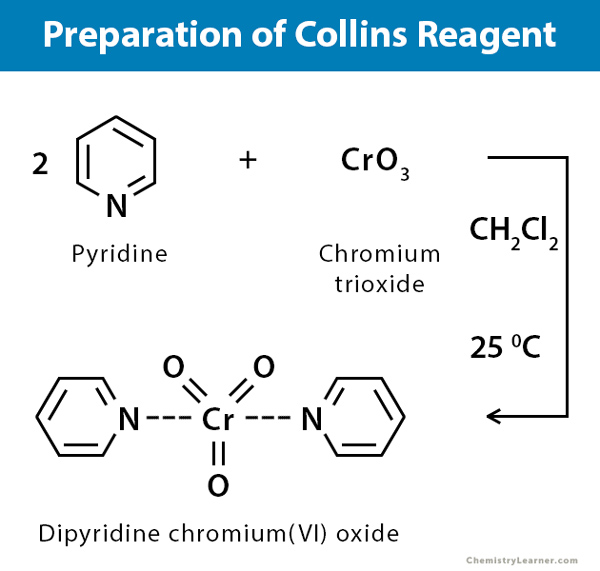
Preparation
The preparation of Collins Reagent can be done by following steps:
- Prepare a stirred solution of two equivalents of pyridine in methylene.
- Add one equivalent of chromium trioxide to the pyridine solution. Be careful during this step, as the reaction is exothermic and hygroscopic, which can cause the mixture to inflame.
- Stir the mixture continuously at 15°C. This will cause the yellow microcrystals to form.
- Keep stirring until the yellow microcrystals change to a deep red macrocrystalline form. This can take several hours.
- Extract the red macro crystals from the mixture.
- Dry the crystals thoroughly.
- Store Collin’s reagent (those red macro crystals) in a safe, dry place for future use.
It’s essential to observe safety precautions while handling the mixture, such as working in a ventilated hood and using appropriate personal protective equipment.
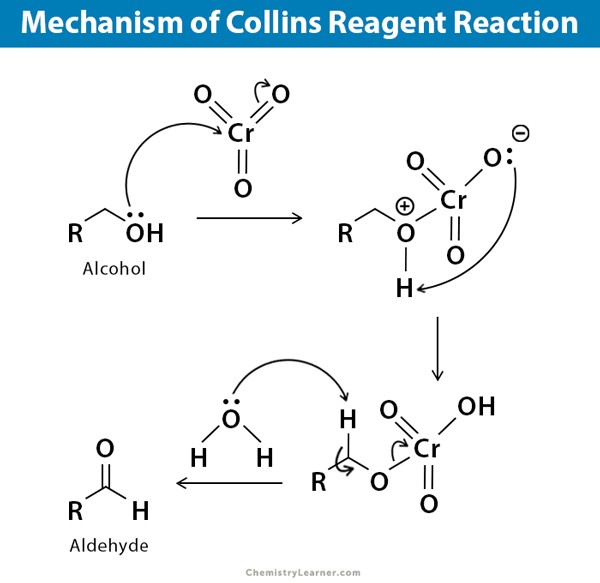
Mechanism
The process of oxidizing alcohols with Collins reagent is commonly referred to as the Collins reagent oxidation reaction.
Its involves a brief and uncomplicated mechanism of oxidation as shown below:
- In the first step chromium atoms present in the starting material CrO3 is reduced from Cr(VI) to Cr(IV).
- In the second step of the mechanism, a C=O bond is formed through an E2 (1, 2 Elimination) reaction.
It is important to note that E2 reactions are commonly known for forming C=C double bonds by eliminating a halide leaving group.
However, in this particular reaction mechanism, an E2 reaction is used to generate a C=O bond by eliminating a reduced metal as a leaving group.
Applications
- Collin’s reagent is utilized for the selective oxidation of primary alcohols into aldehydes, preventing over-oxidation.
- The process known as Collin’s oxidation utilizes Collin’s reagent to effectively oxidize compounds that are sensitive to acid.
- Collin’s reagent is also used as a viable substitution for Jones reagent, Sarett Reagent, and pyridinium chlorochromate (PCC) for the oxidation of secondary alcohols into ketones.
FAQ’S
Collin’s reagent, which consists of dipyridine-complex chromium(VI) oxide in dichloromethane, is utilized to specifically transform primary alcohols into aldehydes through oxidation.
Collins reagent is used to convert a primary alcohol to an aldehyde and a secondary alcohol to a ketone.
C10H10CrN2O3 is the molecular formula of Collin’s reagent.
Related Topics
